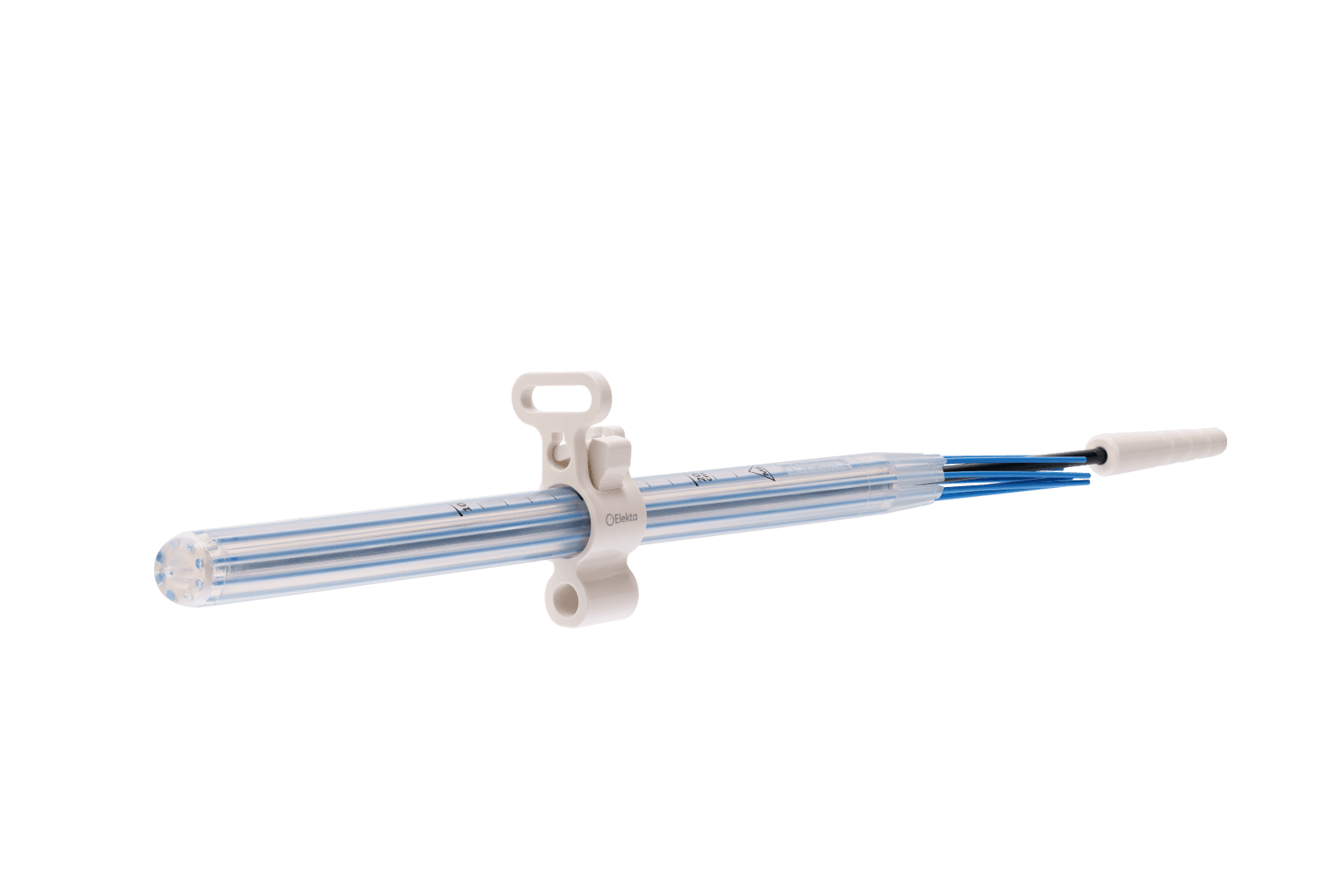
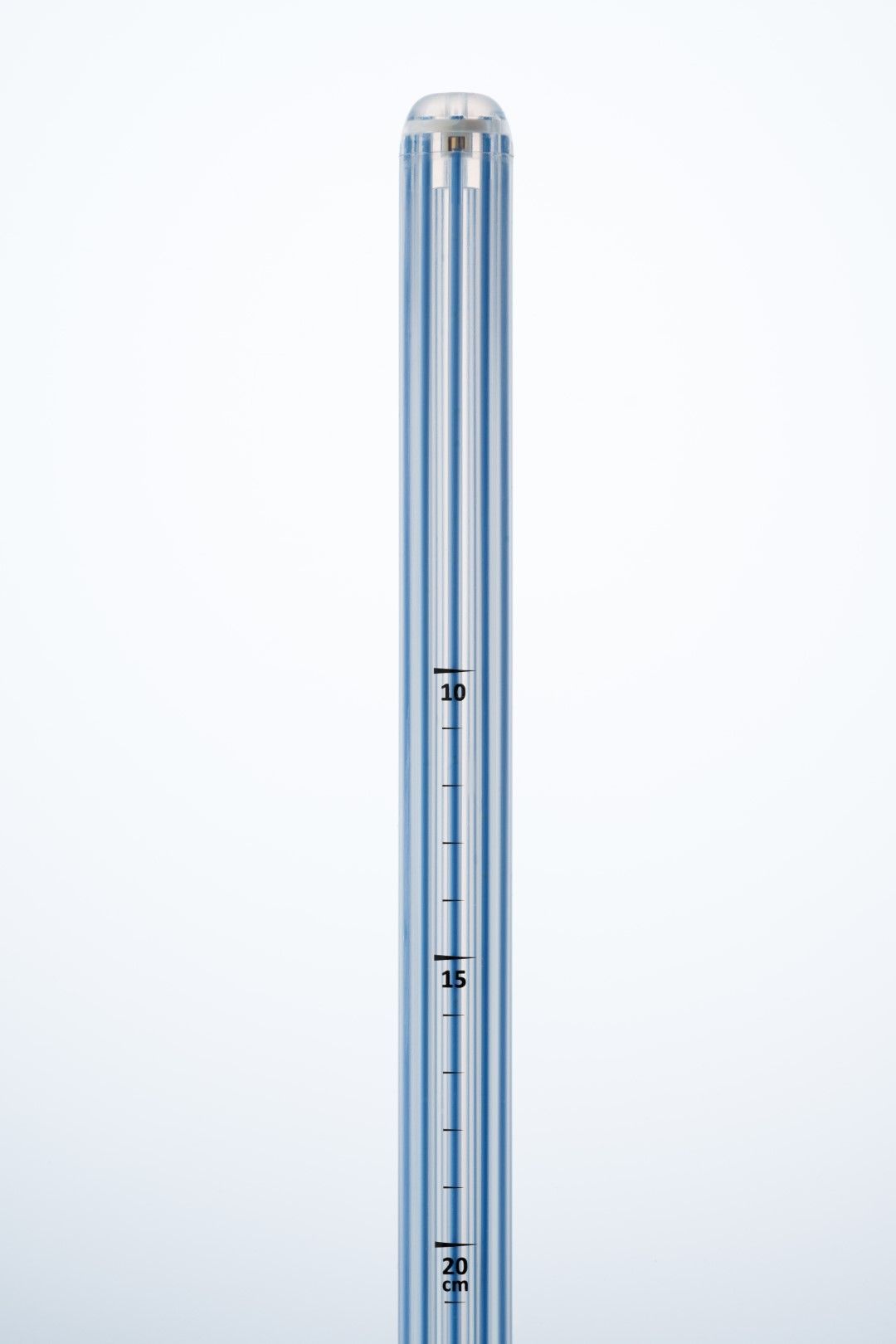
Simplifying HDR endorectal brachytherapy treatment
Single-use applicator, ensuring patient safety
Offering maximum flexibility to minimize patient discomfort.
Easy to use in current clinical workflows
Option to choose from inpatient and outpatient care