Pediatric VMAT total body irradiation (TBI) in the treatment of B-cell acute lymphoblastic leukemia (B-ALL)
Summary
| Patient demographics: | Treatment: |
|
|
| Diagnosis: | Treatment planning and delivery system: |
|
|
Introduction
Total Body Irradiation (TBI) is used in the treatment of certain diseases, such as multiple myeloma, lymphoma, leukemia, and in preparation for bone marrow transplantation (BMT). The purpose of TBI is to destroy residual cancer cells, to destroy the patient’s own bone marrow prior to receiving stem cells, or to prevent donor bone marrow from being rejected (by immunosuppression). TBI has high efficacy, with the ability to penetrate areas, such as the central nervous system (CNS) and testicles, where traditional chemotherapy is ineffective.
Drawbacks of classical TBI techniques include possible dose heterogeneity, a lack of organ-at-risk (OAR) protection, and high delivery uncertainties. The volumetric modulated arc therapy (VMAT) technique was favored for implementing TBI at our hospital due to the accurate and precise dose calculation, OAR protection, and homogenous dose distributions that can be achieved using Monaco’s Monte Carlo dose calculation algorithm.
VMAT TBI was implemented at this hospital in 2015 for 40-50 patients annually. We use VMAT TBI prior to BMT in the treatment of acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) and aplastic anemia. It is applied in patients with brain or testicular involvement and, generally, where chemotherapy alone is insufficient. A TBI regimen is also applied for a second BMT, where the first transplant was unsuccessful.
Based on published guidelines and recommendations1-5, our usual TBI plan delivers 12 Gy in 6 fractions over 3 days, ensuring that the interval between fractions is at least 8 hours. Lung and kidney mean doses are limited to between 9-10 Gy and lens doses to <5 Gy. We aim for target dose homogeneity index (HI) of <1.15.
The following case study demonstrates how OAR-sparing TBI was applied prior to a second BMT in a 6-year-old male patient who experienced early extramedullary relapse (testis) of CALLA-positive B-cell acute lymphoblastic leukemia (B-ALL).
Patient history and diagnosis
This B-ALL patient received his first bone marrow transplant (without TBI) from a fully compatible sibling in March 2017. He experienced an isolated bone marrow relapse 8 months after the initial transplant and was then started on chemotherapy (single dose etoposide 60 mg/kg). While on chemotherapy, the patient experienced a number of complications, including hepatosplenic candidiasis, long QT syndrome, liver dysfunction, cholelithiasis, and BK virus-associated hemorrhagic cystitis. In addition, the patient has congenital kidney atrophy (single kidney). The patient had an orchidectomy in June 2018.
In March 2019, it was decided to perform a second BMT with a TBI regimen. Again, a relative donor was found with 9/10 Human Leukocyte Antigen (HLA) compatibility.
Treatment planning
The patient was positioned using the C-RAD Sentinel surface guidance system and simulated for TBI on a Siemens® Biograph mCT PET-CT scanner (5 mm slice thickness) in the head-first and feet-first positions, since he was over 115 cm in height. Immobilization was achieved with a head-neck mask and the Elekta BodyFIX vacuum bed. Optimum scanning parameters were selected to ensure minimal dose to a pediatric patient.
Contouring of the patient's skin, lenses, kidneys and lungs were performed at a Prosoma workstation. The vacuum bed was included in the external patient contour so that we could calculate the maximum/minimum dose distributions that may occur outside the patient's skin.
Planning was performed using Monaco version 5.11.02 with the Monte Carlo dose calculation algorithm. A VMAT TBI plan was generated for a Versa HD linear accelerator to deliver 12 Gy to the whole body in 6 fractions over 3 days. Dose constraint targets were determined as mean 9-10 Gy for lung, mean 8 Gy for single kidney and mean 4 Gy for lenses. For optimization purposes, the aim was to obtain the desired dose distribution in the PTV, giving priority to lung, kidney and lens constraints.
VMAT plans were prepared in the constrained optimization mode. Homogeneous dose distribution and dose limitations to critical organs were more precisely achieved using multicriteria optimization (MCO). We are able to ensure both planning efficiency and dose distribution quality with MCO-based planning.
Thanks to Elekta Agility’s 40 cm x 40 cm field size, we created the treatment plan using just four isocenters, allowing us to shorten the treatment time as much as possible. The patient volume was divided into four parts in Monaco: PTVHead, PTVThorax, PTVAbdomen and PTVFoot. For this plan, one VMAT field (two arcs) was used at each of the four isocenters. Three isocenters were used in the head-first position (PTVHead, PTVThorax, PTVAbdomen) and a single isocenter was used in the feet-first position (PTVFoot). Overlapping regions of 8 cm are created to avoid hot and cold spots in the intersection zones.
A composite plan was made by fusing the head-first and feet-first plans and using the Monaco Bias Dose feature to manage potential high or low dose points at the junction.
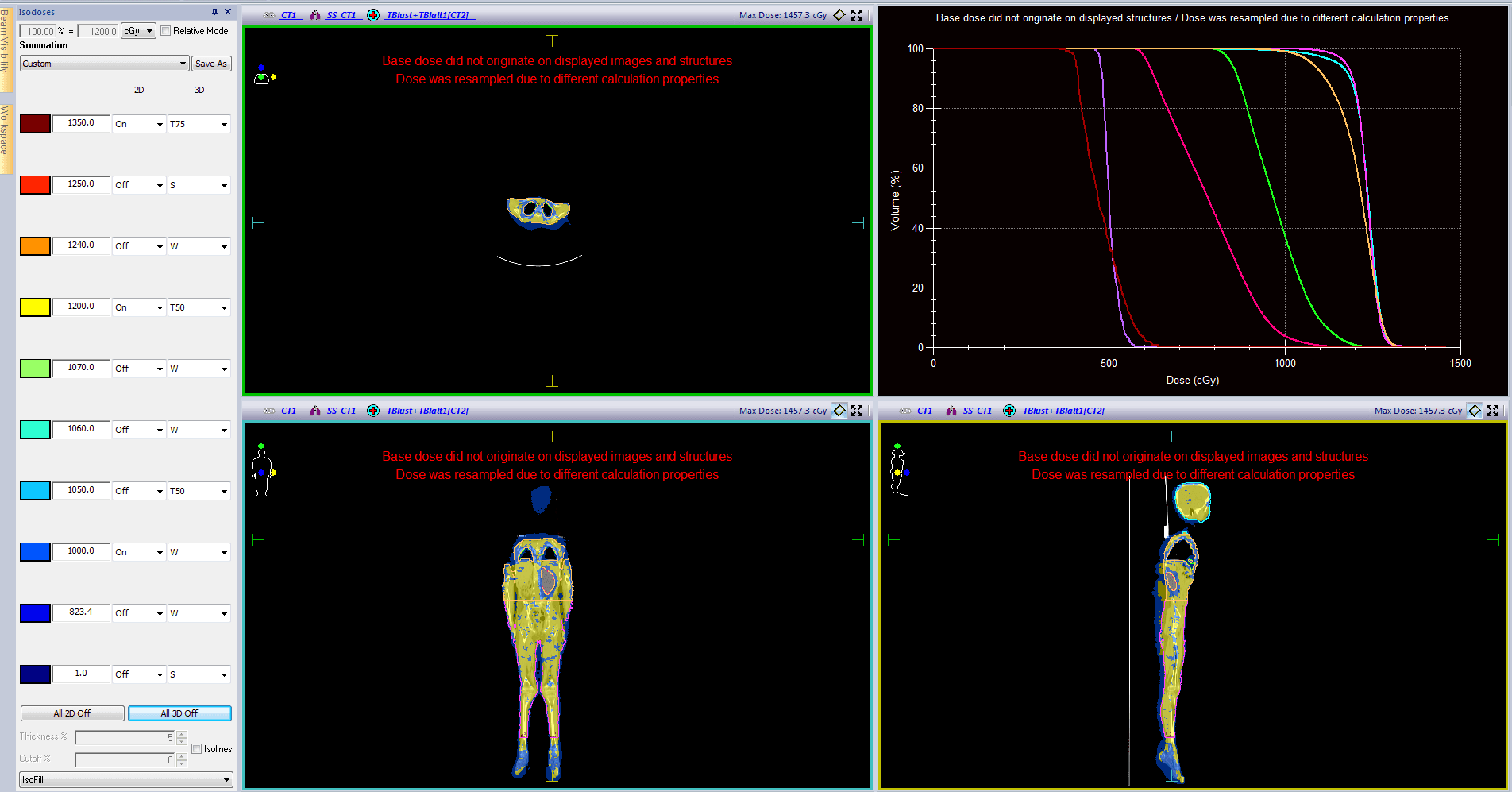
VMAT TBI planning details and dosimetric parameters for this patient are shown in tables 1-3. Dose distributions and DVH curves and shown in figure 1.
Table 1. VMAT TBI planning details
| Isocenters |
|
| VMAT arcs |
|
| Photon beam |
|
| Calculation parameters |
|
| Sequencing parameters |
|
| MLC parameters |
|
Table 2. TBI target volume doses
| D95 (cGy) | DMean (cGy) | DMax (cGy) | HI | |
| PTVHead | 1153 | 1230 | 1379 | 1.09 |
| PTVThorax | 1078 | 1206 | 1393 | 1.19 |
| PTVAbdomen | 1171 | 1230 | 1359 | 1.11 |
| PTVFoot | 1151 | 1213 | 1438 | 1.14 |
Table 3. Critical organ doses
| DMean (cGy) | V60 (cGy) | |
| Lungs | 975 | 942 |
| Kidney (left only) | 786 | 743 |
| Left Lens | 497 | |
| Right Lens | 478 |
Quality assurance
QA for the foot-first single isocenter plan and the head-first combined plan (three isocenters gathered in one plan) was performed using the IBA MatriXX 2D-array. Gamma analysis demonstrated > 90% valid results with criteria of 5% dose difference (DD) and 5 mm distance to agreement (DTA). We also ensure that the average gamma index value is <0.6.
Treatment delivery
The patient was positioned for treatment as for simulation, using C-RAD Sentinel surface image guidance. XVI CBCT scans were performed for each isocenter prior to treatment delivery, as follows:
3D CBCT scanning was performed on the first head-first region and, following corrections, the VMAT field at the first isocenter was delivered. After sliding the table 32 cm longitudinally, the procedure was repeated for the second isocenter and, after sliding the table longitudinally for another 32 cm, it was repeated again for the last head-first isocenter. These three isocenters were treated with a maximum deviation of 2 mm in the longitudinal axis. The patient was then rotated to the foot-first position. A 3D CBCT scan was performed to ensure correct positioning for the fourth isocenter in the lower leg region and the last VMAT field was delivered.
To reduce CBCT dose for pediatric patients, a special CBCT protocol is used: tube potential energy of 100 kV; nominal tube current of 20 mA per frame; S20 collimator; F0 filter; and 183 frames.
The patient was treated twice daily for three days, with an inter-fraction gap of at least 8 hours. The total treatment time for each fraction was approximately 30-35 minutes. Beam on time was 10.13 minutes in the head-first position and 2.91 minutes in the foot-first position. MU values were 2250 in the head-first position and 508 in the foot-first position.
Outcome and follow up
This patient was successfully treated using VMAT TBI, with minimal side effects. Twenty-one months following the second transplant, the patient has experienced no problems or complications.
Discussion and conclusions
Accurate and homogenous dose distributions for VMAT TBI are achieved using the gold standard Monte Carlo dose calculation algorithm in Monaco and the Agility multileaf collimator. In addition, for patient positioning, the use of the C-RAD Sentinel surface tracking system without radiation and the use of low dose CBCT provides maximum setup repeatability and accuracy.
Agility's 40 cm x 40 cm field size, its ability to achieve a virtual 1 mm leaf width with its dynamic leaf guide feature, the long travel distance of the MLC leaves and their ability to form islands on opposite sides simultaneously, are extremely advantageous in achieving the desired dose distributions and OAR protection.
In Monaco, we can achieve homogeneous dose distributions using multiple arcs within a single VMAT field. A composite plan is obtained using the Bias Dose feature in Monaco to prevent hot and cold dose points in the junction areas of the head-first and foot-first plans.
In our experience, VMAT TBI regimens in ALL patients have been very successful in the early and mid-term, with patients experiencing no side effects relating to cataracts, renal function or respiratory function.
Since expected survival rates for patients diagnosed with ALL are good, the long-term side effects of radiation should be considered in patients receiving TBI. More attention should be paid to dose homogeneity, organ preservation and accurate dose calculation, which can be achieved using the VMAT TBI technique. Compared to alternative TBI techniques, this method provides greater accuracy, reliability, and OAR protection, with reduced uncertainties. Homogenous dose delivery can be achieved with minimal side effects, resulting in high BMT success and long-term disease-free survival.
Institution
Institution:
Yeni Yuzyil University Gaziosmanpasa Hospital
Location:
Istanbul, Turkey



References
- ALL SCTped 2012 FORUM. Allogeneic Stem Cell Transplantation in Children and Adolescents with Acute Lymphoblastic Leukaemia - International Therapy Protocol.
- Wong J, Filippi AR, Dabaja B, Yahalom J, Specht L (2018) Total body irradiation: guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys 101(3):521–529.
- Nelligan R, Bailey M, Tran T, Baldwin Z (2015) ACPSEM ROSG TBI working group recommendations for quality assurance in total body irradiation. Aust Phys Eng Sci Med 38(2):205–215.
- VanDyk J, Galvin JM, Glasgow GW, Podgorsak E. editors (1986) AAPM report No. 17. The physical aspects of total and half body photon irradiation. American Inst Phys: New York.
- Quast U (2006) Whole body radiotherapy. J Med Phys 31(1):5–12.