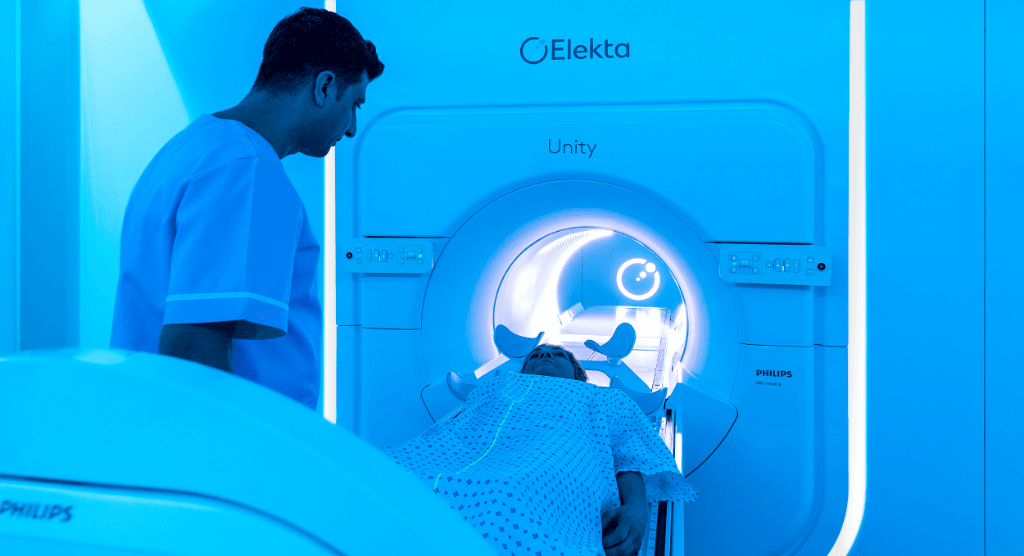
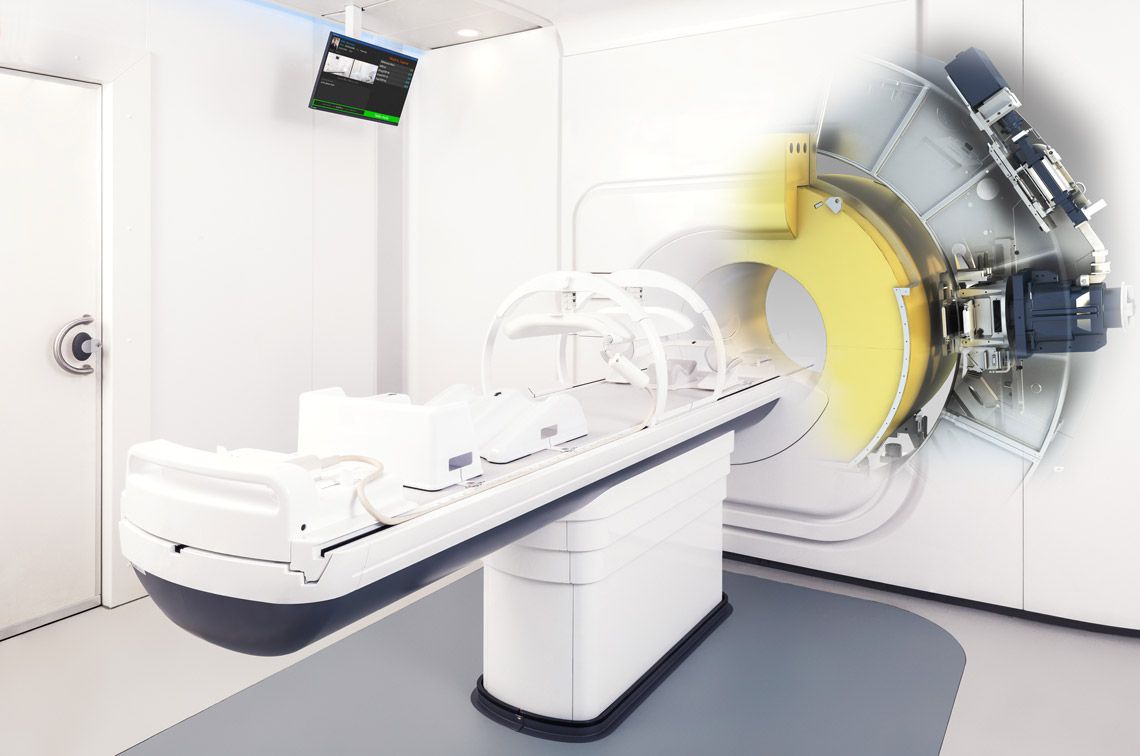
The spectacular evolution of radiation therapy
Former radiographers – now Elekta employees – reflect on the technological transformation of their field
In the period since they began their radiation therapy technologist training in the mid- to late 1990s to today, four former radiation therapists (RTTs) are astonished by how much has changed – from beam shaping and imaging to simulation and planning. In this article, the RTTs, now Elekta employees, reflect on how things used to be and marvel at the dramatic technological progress achieved in a very short time.
Jen Anthony, Customer Engagement Manager

Jen, who began working as an NHS radiographer in 1997, remembers that there were still a few linear accelerators in the department that were analog systems that required manually twisting dials to set the desired dose.
“First we had to calculate the dose using depth dose charts,” she says. “These pre-drawn charts would help us work out the dose for a given size of radiation, and treatment fields were generally delivered in squares or rectangles There were no record and verification systems on these linacs, so all calculations were checked by another radiographer before treatment was delivered. Having digital linacs in the department was seen as a real advance in supporting efficient workflows, allowing us to treat more patients, using techniques such as IMRT and then VMAT to enable faster and more accurate treatment deliveries.
“The introduction of multi-leaf collimators [MLC] was also a major advance and saved a huge amount of time. It was amazing to be able to conform the beam to the shape of the tumour without having to physically add blocks of lead on a shadow tray which had been the case previously.”
In the late 1990s, the introduction of thermoplastic masks radically improved the experience for immobilization of patients with head and neck cancers. Prior to the implementation of thermoplastic masks, patients had to endure a more rigorous mask-making process. The patient’s face would be covered in cold dental alginate to get an accurate impression around their nose, eyes and mouths. Once the alginate set, the patient’s face was wrapped in plaster of paris bandages to ensure the patient could breathe. Once these bandages dried, the impression was lifted off and the patient was free to leave. Over the next week, this impression would be filled with plaster to create a bust of the patient’s head and neck. It would take a few days for the plaster to set, but once this bust was created, a clear perspex sheet was vacuum formed over the bust to create a Perspex mask that the patient would wear during radiotherapy.
“This process was laborious and could take up to 10 days, impacting the start of radiotherapy treatment for a patient” Jen notes. “Today a thermoplastic mask is heated in a warm water bath and then stretched over a patient’s head and neck– the mask is ready in 10 minutes and is a much less distressing experience for patients and they are able to start their treatment sooner. Imaging is another area in which radiotherapy has advanced. When training, Jen had to use X-ray film placed under the patient to verify that treatment was being delivered to the correct area of the body. The film would be processed in a darkroom which took time and negated the accuracy of this activity in hindsight.
“A few years after I started training, digital imaging was introduced,” Jen says. The imaging arm had to be wheeled out to the linac on a trolley and then cranked onto the linac. The introduction of digital imaging was revolutionary because there was an instant image on a screen which enabled accurate clinical decisions.
“It is a privilege to have been part of this revolutionary phase in radiotherapy; although the processes we undertook in the past seem old-fashioned now, the profession was always keen to improve and move the needle forward.”
“It is a privilege to have been part of this revolutionary phase in radiotherapy; although the processes we undertook in the past seem old-fashioned now, the profession was always keen to improve and move the needle forward,” she continues. “A lot of the exciting innovations implemented coincided with advances in technology and development of new materials in everyday life. It is also important to recognize how our clinical collaborations drive us to innovate and put our patients’ experience and outcomes at the heart of everything we do.”
Robin McCaw, Director, Validation & Pilot Release

In the mid-1990s, being a radiologic technologist meant a lot of manual labor, according to Robin McCaw.
“We used heavy, metallic Cerrobend blocks to shape the radiation beam, because MLCs were not around, nor were dynamic wedges. We had to use manual wedges,” she remembers. “All the manual work involved in radiation therapy meant we were able to eat all the goodies patients brought us and not gain weight!”
The patient journey at this time included a conventional simulation that entailed tattooing the patient, who would return a few days later for pre-treatment imaging and then usually treatment the same day. Patients would commonly have multiple weeks of treatment with weekly imaging, Robin explains.
“The patient experience has improved tremendously since then,” she says. “Technological improvements – including MLC, dynamic wedges, EPID, cone beam CT and MR imaging – have come together to assist radiation therapists in the delivery of treatment that is faster, more precise and results in less normal tissue irradiation. The number of treatment sessions required has decreased in many cases and imaging can take place daily instead of weekly. These changes in the patient journey are enabling patients to complete their treatment and get back to normal life quicker.
“It’s gratifying to know our profession is not resting on its laurels and instead is constantly looking for ways to improve the treatment experience and outcomes for patients.”
“It’s gratifying to know our profession is not resting on its laurels and instead is constantly looking for ways to improve the treatment experience and outcomes for patients,” Robin continues. “Likewise, it is a great feeling to work for a company that has introduced or enhanced some of these innovations.”
Liz Raspa, Marketing Director, Region Europe

To Liz Raspa, the linacs of the mid- to late 1990s seem rather primitive now.
“The first machine I ever switched on as a trainee therapeutic radiographer was an SL75 that had no computer system,” she recollects. “It had little switches that you flicked to set the dose to deliver and a pedestal controller that you pushed around the floor to move the gantry and collimator.”
On-treatment verification images were taken mainly using X-ray film in a holder, she adds. The more “cutting edge” machines had an imaging device that was hooked onto the machine when an operator wanted to use it. Treatment planning was carried out using a simulator, which was essentially a diagnostic X-ray system in a machine that mimicked the movements of a linac.
“Improvements in on-treatment imaging have been particularly significant as they have taken the blinkers off and enabled therapeutic radiographers to have a more accurate picture of what we are treating just before or even during treatment,” Liz observes. “Cone beam CT was a great start to this and helped radiographers understand what happens to tumors in different parts of the body in relation to motion. Magnetic resonance-guided radiotherapy is taking this to the next level.”
While the equipment used in radiotherapy has evolved and advanced over time, the patient journey arguably has remained similar in terms of the stages required to get from diagnosis to treatment and follow-up, she says.
“Not only have treatments changed drastically, for example, how we can conform the beam to the tumor to spare healthy tissue, but advances in radiotherapy techniques also now mean that treatment courses are shorter.”
“Undoubtedly, the patient experience has improved with technological advancements,” Liz notes. “Not only have treatments changed drastically, for example, how we can conform the beam to the tumor to spare healthy tissue, but advances in radiotherapy techniques also now mean that treatment courses are shorter. Shorter treatment courses help save and increase the efficiency of precious healthcare resources, but also bring numerous advantages to the patient in terms of not having to attend the radiotherapy clinic as many times to complete their treatment. Aside from convenience, it also makes it easier for patients to mentally prepare for and undergo treatment when a course of radiotherapy treatment is shorter.”
Matt Vigar, Vice President, Head of Marketing & Sales, Oncology Informatics
It wasn’t until Matt’s last hospital placement during his training in Australia 20 years ago that he first saw and used electronic portal imaging.

“We had used basic simulator-based CT scans to plan most patients, but radical patients were sent to medical imaging for a planning CT,” he says. “Things have significantly progressed in imaging technology since then, although I never saw an Elekta machine before I arrived in the United Kingdom in 2006.”
These days, patient treatment is adapted to a greater extent and are more precise due to new imaging protocols, such as CBCT and MR-guided radiation therapy, Matt says.
“The patient journey hasn’t changed too much since my training days, but today it does involve more multi-disciplinary treatment decisions, immunotherapy, for example, and changes in chemotherapy scheduling have greater interdependencies,” he observes. “The patient experience has improved in line with progress in imaging technology. Treatment times are shorter in most cases, more accurate with greater visualization of tumor shape, and we can incorporate adaptive changes during treatment depending on the patient response.”
At the start of Matt’s career, SBRT was not widely implemented and then only in special centers.
“Today, patients can get a far superior treatment than was offered 20 years ago and it is accessible at local clinics, as well.”
“Today, patients can get a far superior treatment than was offered 20 years ago and it is accessible at local clinics, as well,” Matt says. “However, there are two technological advances that I believe have had the most impact on radiation therapy and the work of radiographers. First, MLCs – such as Elekta’s Agility™ MLC – have increased the speed of beam delivery and the accuracy of treatments. Second, the support of these MLCs through imaging developments, such as bringing MRI into the treatment room. Both of these innovations have greatly impacted many types of patients, as they not only allow us to treat more common tumor types faster and more accurately than ever before, but they also enable us to identify and treat harder-to-see tumors.
Matt thinks there are still more innovations coming on the horizon.
“I expect artificial intelligence will drive even more advances in the patient experience and outcomes in the years to come,” he says.