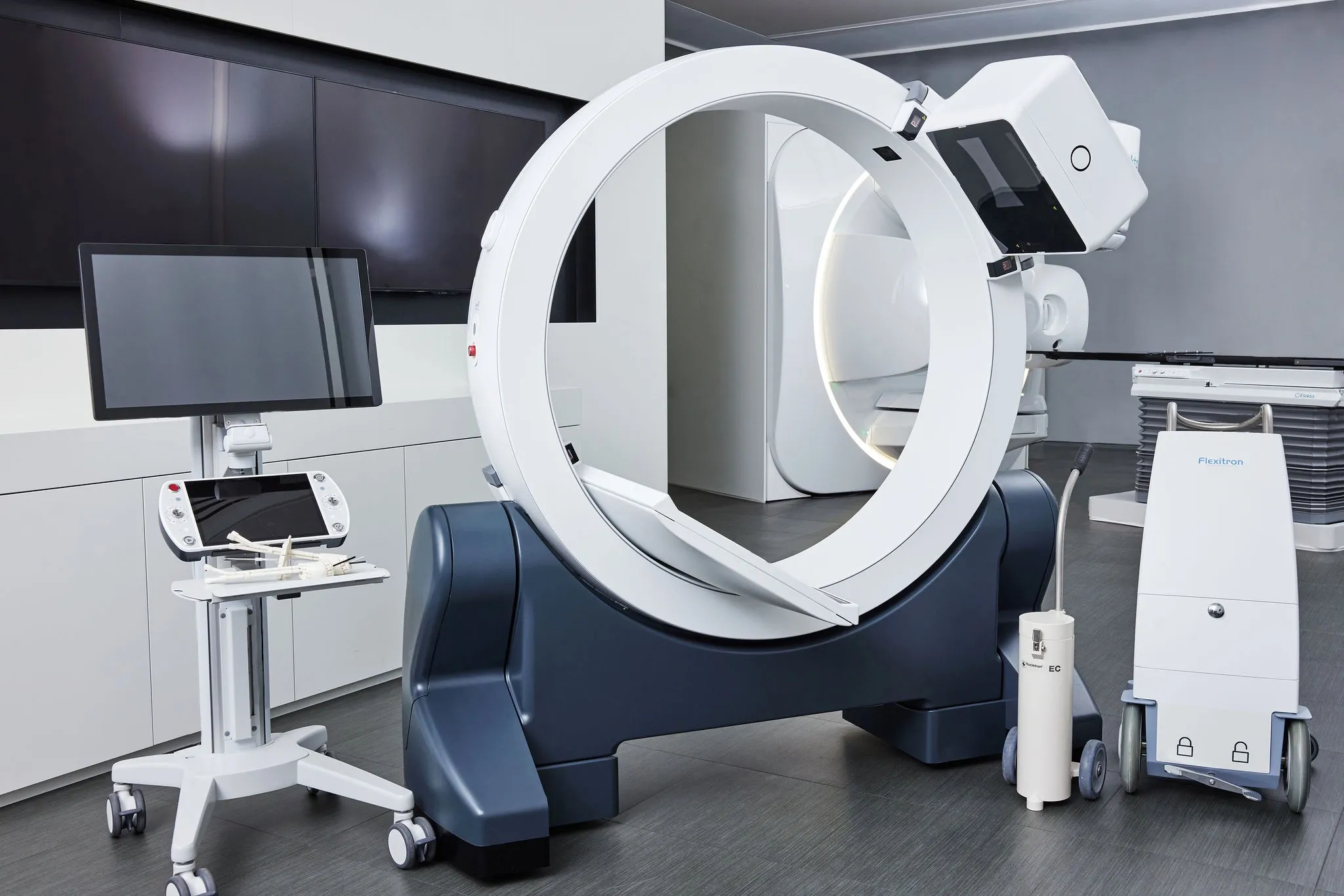
The enduring role of radiation therapy in cervical cancer treatment

High dose, image-guided brachytherapy an indispensable component for treating locally advanced cervical cancer at Medical University of Vienna
Cervical cancer is not only among the most preventable types of cancer – due to the uptake of screening and the human papilloma virus (HPV) vaccination – but it’s also one of the most treatable when detected early. Despite that, the disease remains the fourth most common cause of cancer incidence and mortality globally and is a significant public health problem.1
“IGBT [image-guided brachytherapy] is an essential component of definitive radiotherapy and should not be replaced with an external boost (photon or proton).”
In an effort to improve care for women with gynecological cancers, a panel of experts from the European Society of Gynecology, alongside the European Society for Radiotherapy and Oncology and the European Society of Pathology, recently indicated that for locally advanced cervical cancer (LACC, stages T1b3-T4a): “IGBT [image-guided brachytherapy] is an essential component of definitive radiotherapy and should not be replaced with an external boost (photon or proton). If BT [brachytherapy] is not available, patients should be referred to a center where this can be done.”2
In key LACC studies, IGBT, combined with chemoradiation, has been shown to result in 92 percent actuarial local control and 87 percent actuarial pelvic control at 5-year followup3-4, with both investigations reporting limited severe morbidity. These results represent a standard for advanced clinical practice and future clinical studies in LACC.
An evolving technique

Alina Emiliana Sturdza, MD, FRCPC, a radiation oncologist at the Medical University of Vienna, and ESMO representative for the World Health Organization’s Cervical Cancer Elimination Initiative, has presided over the evolution of brachytherapy, a period in which the modality transformed from a 2D x-ray method to its current, advanced 3D image-guided form employing MRI or CT.
Due to limitations in the ability to visualize cervical soft tissues and organs-at-risk (OAR), 2D brachytherapy was a less personalized treatment, she says.
“At that time, the only images available for BT were x-rays, so you can see only the applicator in the best case, not the target or OAR,” she says. “That meant nearly all tumors were treated the same, regardless of their size and we just relied on the pear shape of the prescribed treatment dose to point A encompassing the cervix and tumor, while using a fixed dose. Essentially, the isodose line was almost the same for everyone with slight variation in length and width, depending on the dimensions of the applicator.
“The use of MRI in IGBT changed all of that, as MRI is the imaging modality of choice for gynecological malignancies,” Dr. Sturdza continues. “You can see the applicator and the tumor exceptionally well, in addition to the OAR. Accordingly, clinicians know precisely where to apply the dose and they can adapt the application, and therefore the plan to cover the whole tumor, while also sparing OAR.”
MRI-guided IGBT at the Medical University of Vienna
The Medical University of Vienna performs IGBT for about 125 patients each year, approximately half of whom will receive the external beam radiation therapy (EBRT) component of their treatment with a linac at their local hospital and referred to Vienna for the brachytherapy boost component.
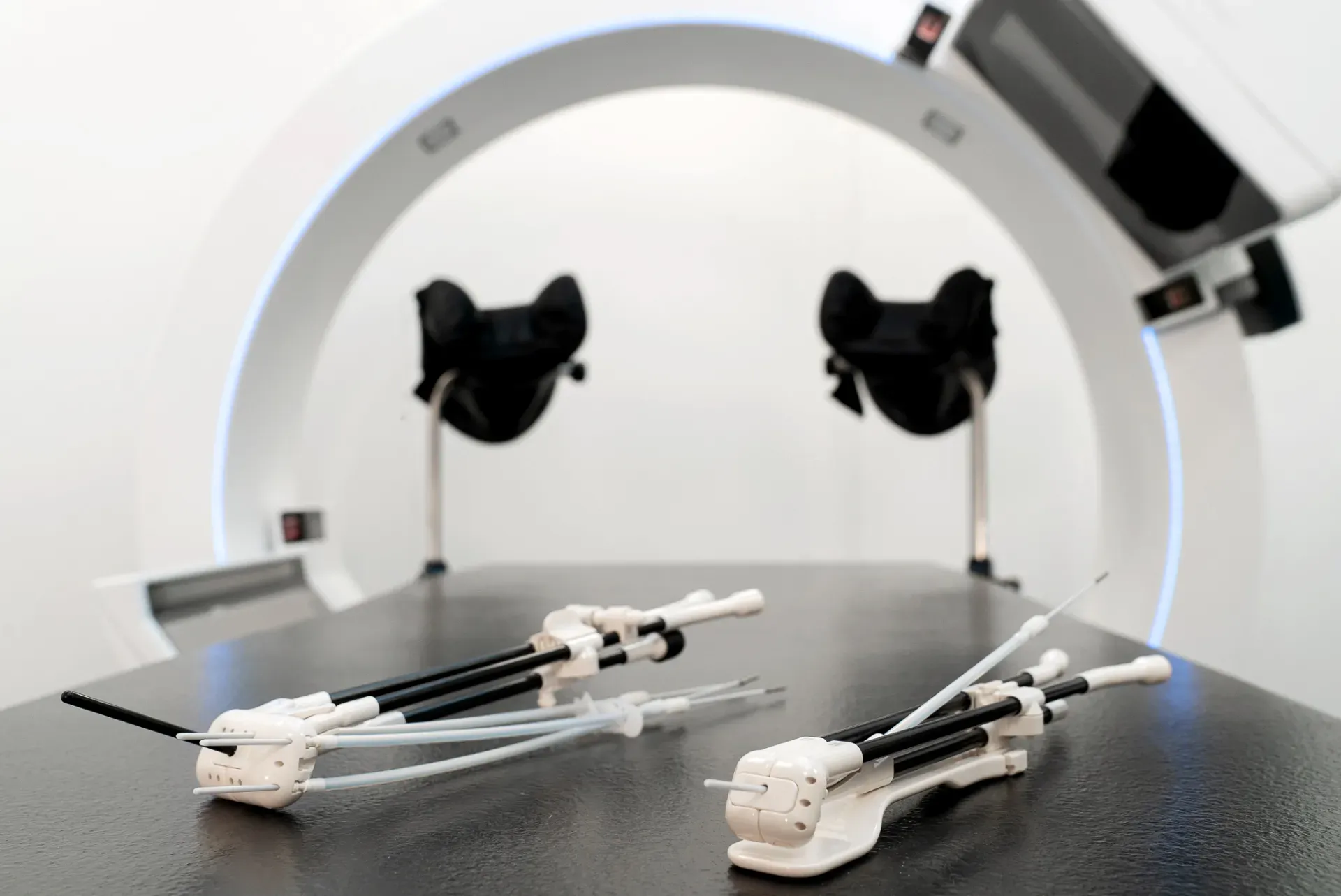
The center employs a 1.5T MRI unit for contouring the high-risk clinical target volume (HRCTV) and/or the intermediate-risk CTV (IRCTV). EBRT is delivered with an Elekta Versa HD™ linear accelerator and brachytherapy is performed with the Elekta Flexitron® afterloader. For intracavitary/interstitial brachytherapy, clinicians use Elekta’s Venezia™ advanced gynecological applicator for the majority of cases.
“…the combination of advanced EBRT, followed by the high-dose brachytherapy boost and a sophisticated applicator allowing both intracavitary and interstitial methods represents the best possible treatment for LACC.”
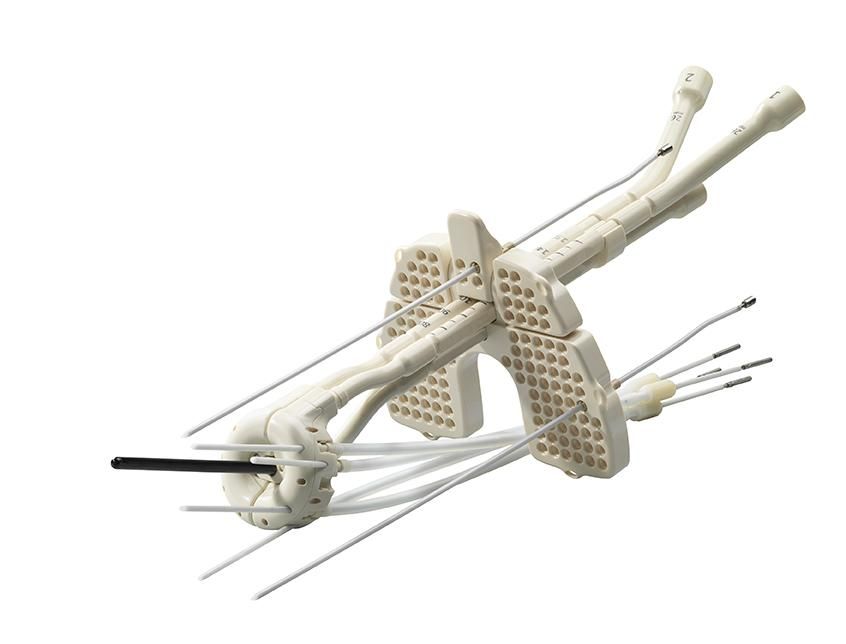
“In my view, the combination of advanced EBRT, followed by the high-dose brachytherapy boost and a sophisticated applicator allowing both intracavitary and interstitial methods represents the best possible treatment for LACC,” she says. “The exceptional local control and pelvic control outcomes3-4 we have seen at our center is strong clinical evidence of that.”
To treat macro and potential microscopic disease at diagnosis (tumor, uterine tract and the pelvic lymph nodes), patients receive chemoradiation – 25 fractions of EBRT to 45 Gy concurrently with a once-weekly infusion of cisplatin for at least five cycles. In the last week of chemoradiation, or after this treatment component, patients receive brachytherapy.
“The brachytherapy boost is used to control volumes that contain a macroscopic tumor rest at the time of brachytherapy, in addition to the whole cervix and areas with potential residual microscopic disease in the region where the tumor was present at diagnosis,” Dr. Sturdza explains.
Before brachytherapy, patients have a gynecological examination and an MRI to evaluate the tumor response to chemoradiation and to develop a pre-plan, including choosing the type of applicator needed and where interstitial needles should be placed.
“Even if the tumor is not very large, we may still use interstitial needles to spare organs-at-risk,” she observes. “The loading pattern starts with a standard intracavitary tandem and ring, which is then adapted to cover the volume of interest, also including the use of the inserted needles.”
Patients receive two fractions of HDR brachytherapy (PDR in some cases), keeping the patient overnight with the Venezia applicator in situ. This type of application is usually performed twice, with the patient generally receiving four HDR fractions.
“In between the two applications, there is a two to four day pause. The total brachytherapy boost dose is 45-50 Gy EQD2,” she says.
Clinicians aim to complete the treatment – from the start of chemoradiation to the completion of brachytherapy – in 45 days. Six to seven weeks of treatment (chemoradiation + BT boost) can be a daunting prospect for some patients – enough for them to decline either chemotherapy or the BT phase, Dr. Sturdza notes. However, she has a way that helps persuade reluctant patients.
“We developed an app that I use in clinical practice to demonstrate to patients how useful it is to do the treatment to the end in order to have the best chance of surviving5,” she says. “I show them the curves and what the probability of being alive in five years would be if they do it. It’s very compelling and it gives them the hope and the courage to go through with the entire treatment.”
Clinical cases
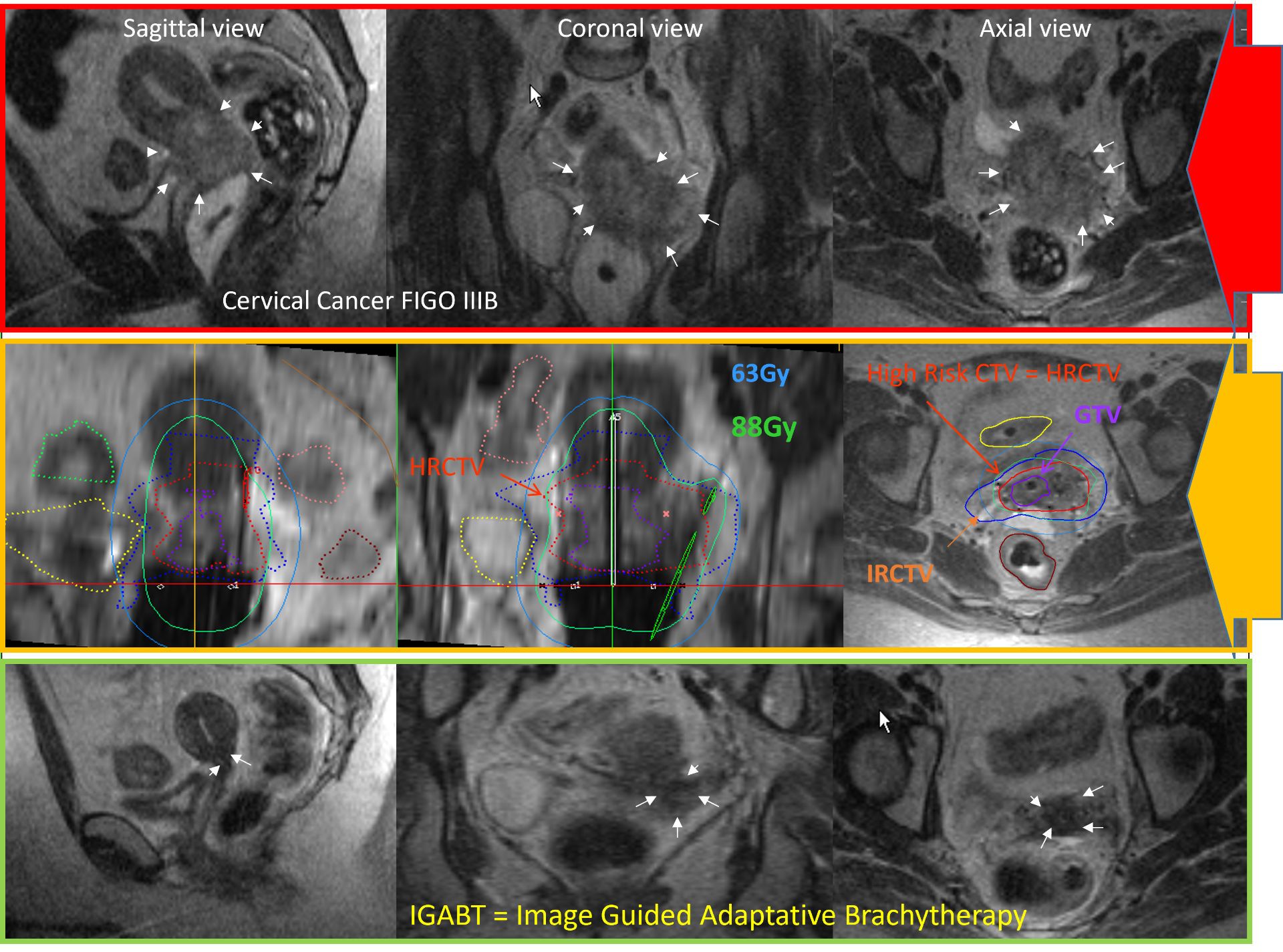
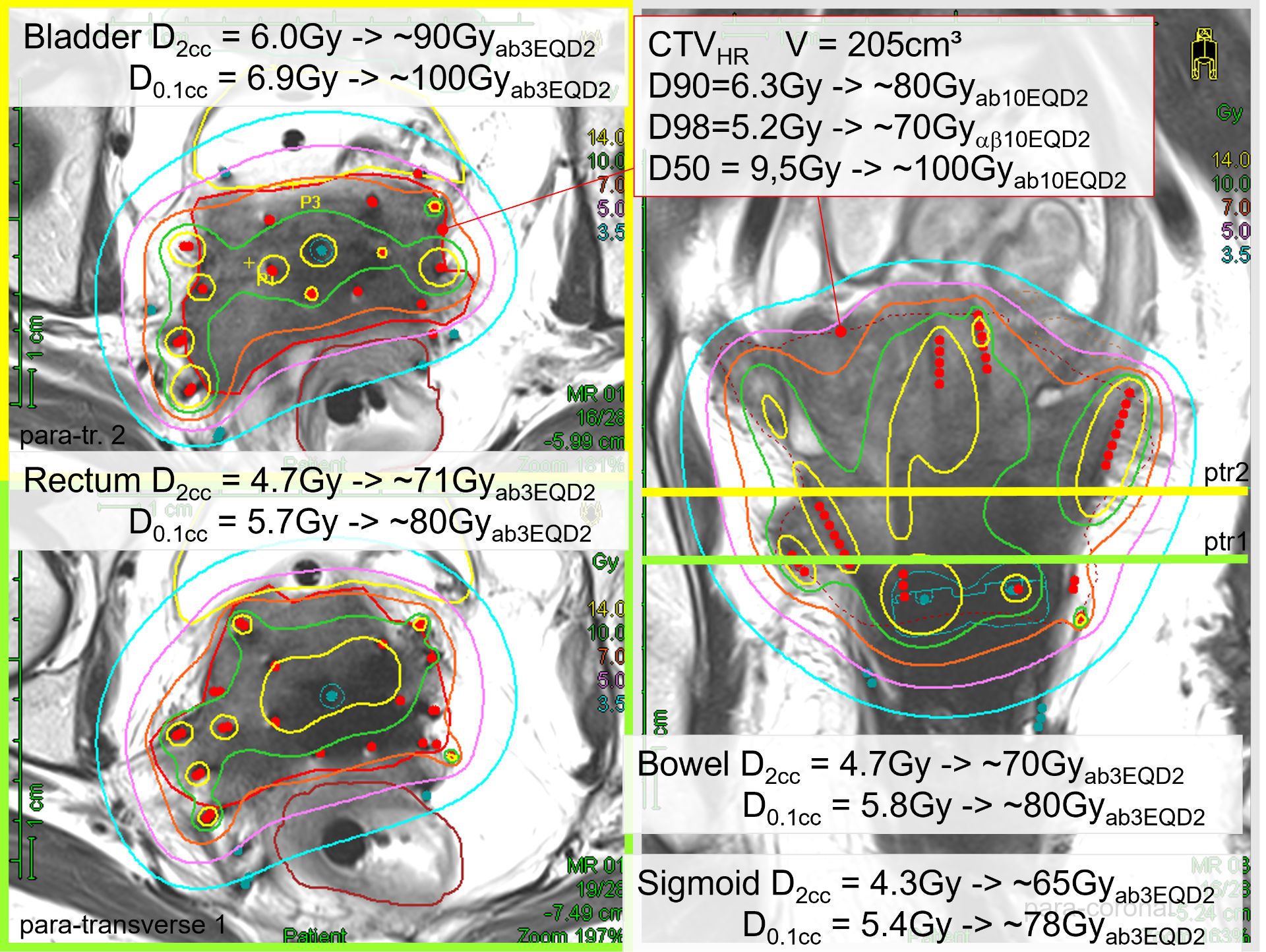
Dr. Sturdza cites a successful 2011 brachytherapy case of a patient with a challenging T1b3 tumor with expansion to the pelvic wall (Figure 1).
“She just visited me 12 years after treatment and she is healthy and enjoying life.”
“She just visited me 12 years after treatment and she is healthy and enjoying life,” she says. Another difficult case was a patient with a T4a tumor with bladder infiltration. (Figure 2).
“There are still centers that prefer not to treat the patients with bladder infiltration with radical intent and consider these palliative cases, because of the high risk of a vesicovaginal fistula,” Dr. Sturdza notes. “We do treat these cases with curative intent here, and we showed in the RetroEMBRACE3 and EMBRACE4 studies that these T4a patients have an overall survival of 52 percent at five years.”
Brachytherapy implementation support
Despite impressive five-year local control rates, cervical brachytherapy has been underutilized internationally due to various factors, including limited exposure to the procedure during residency training; difficulty in skills maintenance; lack of supportive infrastructure; low volume treatment centers; limitations in advanced imaging techniques; and financial constraints.6-12
If centers can surmount these barriers, assistance in learning and performing the complex tasks involved in cervical brachytherapy is available, according to Dr. Sturdza.
“For radiotherapy departments that are just beginning a cervical brachytherapy service and encounter complicated cases, they should ask for help from, or refer to, experienced centers, which I think will be more than willing to assist them,” she says. “In addition, Elekta’s BrachyAcademy provides workshops that give clinicians tremendous confidence in performing brachytherapy.”
Learn more about Elekta’s brachytherapy solutions.
References
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020;8:e191–203. 10.1016/S2214-109X(19)30482-6.
- Cibula D, Raspollini MR, Planchamp F, et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer - Update 2023. Int J Gynecol Cancer. 2023 May 1;33(5):649-666. doi: 10.1136/ijgc-2023-004429. PMID: 37127326; PMCID: PMC10176411.
- Sturdza A, Pötter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016 Sep;120(3):428-433. doi: 10.1016/j.radonc.2016.03.011. Epub 2016 Apr 29. PMID: 27134181.
- Pötter R, Tanderup K, Schmid MP, et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol. 2021 Apr;22(4):538-547. doi: 10.1016/S1470-2045(20)30753-1. PMID: 33794207.
- Sturdza AE, Pötter R, Kossmeier M, et al. Nomogram predicting overall survival in patients with locally advanced cervical cancer treated with radiochemotherapy including image-guided brachytherapy: A Retro-EMBRACE study. Int J Radiat Oncol Biol Phys. 2021 Sep 1;111(1):168-177. doi: 10.1016/j.ijrobp.2021.04.022. Epub 2021 Apr 29.PMID: 33932530
- LaVigne AW, Triedman SA, Randall TC, et al. Cervical cancer in low and middle income countries: addressing barriers to radiotherapy delivery. Gynecol Oncol Rep. 2017;22:16–20.
- Ma TM, Harkenrider MM, Yashar CM, et al. Understanding the underutilization of cervical brachytherapy for locally advanced cervical cancer. Brachytherapy. 2019;18(3):361–369.
- Grover S, Xu MJ, Yeager A, et al. A systematic review of radiotherapy capacity in low- and middle-income Countries. Front Oncol. [Internet]. 2015.
- Petereit DG, Frank SJ, Viswanathan AN, et al. Brachytherapy: where has it gone? J Clin Oncol. 2015;33(9):980–982.
- Thaker NG, Meghani R, Wilson C, et al. Impact of the radiation oncology alternative payment model on brachytherapy reimbursement. Brachytherapy. 2021;S1538–4721(21):00099-4.
- Sturdza AE, Stephanides M, Jurgenliemk-Schulz I, et al. Brachytherapy training survey among radiation oncology residents in Europe. Radiother Oncol. 2022 Dec;177:172-178. doi: 10.1016/j.radonc.2022.10.030. Epub 2022 Oct 31.PMID: 36328092
- Mittal P, Chopra A, Kamrava M, et al. Brachytherapy training in India: Results from the GEC-ESTRO-India survey. Brachytherapy. 2023 Jul-Aug;22(4):562-569. doi: 10.1016/j.brachy.2023.04.002. Epub 2023 May 14.PMID: 37193616
LARBX240112