Technique shows promise of earlier quantification of a brain tumor’s responsiveness to radiotherapy
Researchers debut advanced CEST MRI sequence on Elekta Unity MR-Linac
Investigators at Sunnybrook Research Institute (Toronto, Canada) are the world’s first to employ – on their 1.5T Elekta Unity system – a sophisticated MRI technique called chemical exchange saturation transfer (CEST) for patients with central nervous system (CNS) tumors. Using the CEST MRI pulse sequence periodically during a radiotherapy course could help clinicians better understand how aggressive, malignant CNS tumors (i.e., GBM, astrocytoma) are responding to irradiation, enabling them to modify therapy to improve outcomes. Elekta spoke with the principal investigator of this Sunnybrook study, Angus Lau, PhD, and research associate, Rachel Chan, PhD, about their team’s CEST/Unity study1 involving 54 CNS tumor patients receiving radiotherapy.

Study design: CNS patients were treated with up to 30 fractions (total dose ≤ 60 Gy) using Elekta Unity. CEST scans were obtained in the subjects at one or more time points during treatment and 3 CEST metrics were quantified. The signal was investigated between tumor and white matter, across time, and across disease categories, including high- and low-grade tumors.1
Elekta: Why is it clinically challenging to identify which CNS tumors are responsive to radiotherapy?

Dr. Lau: Active tumors may be obscured on the images by the immediate effects of treatment, inflammation or pseudoprogression. Determining whether or not a tumor is responsive currently requires waiting months to see if there is a change in tumor size. This is why we acquire a battery of MRI scans, including CEST, in CNS tumor patients throughout their treatment: to see if any of the images can be used to predict tumor response.
Elekta: How can CEST help?
Dr. Chan: We know from our previous studies2-8 that CEST can detect metabolic changes that precede changes in tumor size. Solving this challenge would allow early modification of the treatment in the radiotherapy course, potentially improving patient outcomes in a personalized approach.
Elekta: How is CEST different from traditional MRI pulse sequences?
Dr. Lau: A typical MRI scan provides excellent soft-tissue contrast, but provides little information on physiological processes, such as metabolism. CEST is sensitive to metabolic and molecular information, allowing us to non-invasively image cellular processes. CEST can be made to detect a wide range of molecular groups found in tumors. A popular CEST technique is called APT (see sidebar), which specifically probes the amide chemical groups on proteins.
Elekta: Your center’s CEST study was the first to use the technique on an MR-Linac. Why is that significant?
Dr. Chan: The treatment plan and radiation doses in standard radiotherapy are normally fixed throughout the treatment cycle. The main advantage with MR-guided radiotherapy is that we have the ability to adjust the radiation dose daily based on observable biological changes. This can be done, for example, by escalating the dose to certain parts of the tumor that may be more aggressive – and do this before the end of the treatment cycle.
Elekta: Couldn’t this simply be done with a regular diagnostic MRI system?
Dr. Lau: It’s a suitable alternative. But while diagnostic MRIs are more readily available, it would be logistically more challenging, and frequent monitoring on a different machine would be inconvenient for patients. On the MR-Linac, contouring and subsequent irradiation could be done with the patient in the exact same position, potentially enabling more accurate, smaller margins, or margins that reflect biological changes based on CEST and other functional imaging.
Elekta: What did the three CEST metrics (MTRamide, MTRNOE, asymmetry) that the Sunnybrook study employed tell you about CNS tumors and contralateral normal appearing white matter (cNAWM)?
Dr. Chan: Higher CEST asymmetry has been attributed to increased metabolism or possibly increased levels of soluble proteins and peptides. Conversely, the MTR signal in tumor is typically lower than that of cNAWM. It is encouraging that on the Elekta Unity, we see trends that are consistent with those of other studies of CEST on diagnostic scanners – namely that CEST metrics change over time and show differences between tumor grades. In addition to the gross tumor volume (GTV) tumor signal, it is useful to quantify the signal of the cNAWM, which also has been shown to be altered in patients with brain cancer.4
Elekta: Regarding the results of your study, what did you find about CNS tumor and white matter contrast, CEST signal changes over time, and the differences between high-grade and low-grade tumors?
Dr. Lau: We built on previous studies, including those from our institution2-8, that have reported similar results at higher field strengths. For example, previous studies have shown that CEST asymmetry correlates with the level of cell proliferation, which is increased in tumors of higher grades.9-10 CEST, in some cases, can also predict treatment outcome. A decrease in the CEST signal during treatment has been correlated with tumor shrinkage.
We also confirmed that the MR-Linac is sensitive enough to detect tumor/white matter contrast and signal changes during treatment, which have been shown by previous studies at higher field strengths on diagnostic MRI systems. Signal changes over time could be reflecting a number of treatment-induced effects, including edema, apoptosis and other microstructural or metabolic changes.
Elekta: Given these results, how might CEST imaging on the MR-Linac help physicians adapt radiotherapy?
Dr. Chan: An ideal scenario might look like the following: Patients are scanned during treatment on the MR-Linac with a set of MRI pulse sequences, including CEST, which have been shown to detect tumor response early during therapy. On each day of radiation, we assess whether the treatment is having the intended effect on the tumor, using all imaging and clinical data. If the tumor is responding – for example, showing decreased metabolism as determined by CEST – then we continue the treatment and we keep monitoring every day. If the tumor is unresponsive, then possibly some intervention is needed, such as increasing the radiation dose to aggressive tumor regions or including alternative therapy schemes.
Elekta: When do you predict CEST imaging on an MR-Linac, such as Elekta Unity, could become clinically routine?
Dr. Lau: It’s already possible to routinely acquire CEST images on Elekta Unity at our center, and the pulse sequence is amenable to implementation at other centers. Before adaptation based on CEST, however, we first need to find strong evidence that the CEST images that we acquired in our initial group of 54 GBM patients can be used to predict clinical outcomes and future regions of tumor progression.
Amide Proton Transfer (APT) MRI reflects concentration of endogenous proteins in brain tumor11
In APT-weighted imaging and other CEST methods, the MRI signal is generated by a mechanism different from that of basic MRI. These CEST techniques are based on the chemical exchange of hydrogen atoms. The signal of amide protons of peptide bonds in proteins is too low to be measured with normal MRI. The hydrogen (proton) exchange between protein amide groups and surrounding water allows a different way to measure these amide protons.
In APT, an RF prepulse (saturation pulse) at the amide hydrogen’s frequency is given to attenuate its MR signal. Because the amide group and water continually exchange hydrogen atoms, the number of saturated protons will build up in water, so that the measured water signal will become lower. The change of the MRI signal of water provides an indirect way to measure the presence of amide. APT images are usually presented as color maps, created by using an asymmetry calculation so that presence of APT is shown as a positive-colored signal.
Studies have shown that the APT signal correlates with protein levels and is related to cell proliferation, and thus, the APT signal strength reflects the grade of malignant tumors.10-11 APT contrast can potentially highlight tumors that wouldn’t be seen otherwise.
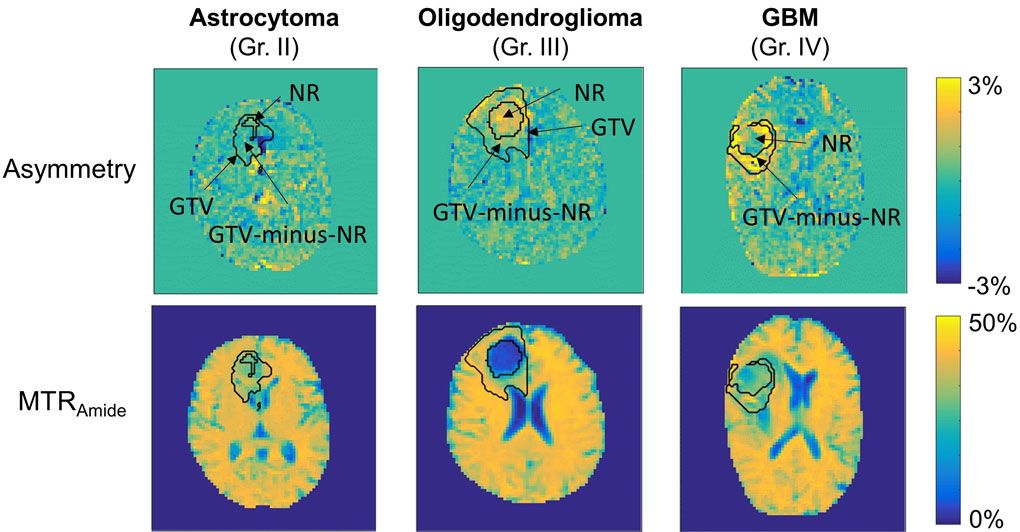
Example asymmetry and MTRAmide maps are shown for three patients, including a WHO grade II astrocytoma, grade III oligodendroglioma, and glioblastoma (GBM). “NR” represents the necrotic or surgically-resected cavities that have been removed from the GTV (modified to exclude any ventricle regions) to create the “GTV-minus-NR” region. Higher CEST asymmetry can be seen in higher-grade tumors. The magnetization transfer ratio (MTR) maps are less noisy and also show CEST image contrast.
Learn more about Elekta Unity.
References
- Chan RW, Lawrence LSP, Oglesby RT, et al. Chemical exchange saturation transfer MRI in central nervous system tumours on a 1.5 T MR-Linac. Radiother Oncol. 2021 Sep;162:140-149.
- Mehrabian H, Myrehaug S, Soliman H, Sahgal A, Stanisz GJ. Evaluation of glioblastoma response to therapy with chemical exchange saturation transfer. Int J Radiat Oncol Biol Phys 2018;101:713–23.
- Mehrabian H, Myrehaug S, Soliman H, Sahgal A, Stanisz GJ. Quantitative Magnetization Transfer in Monitoring Glioblastoma (GBM) Response to Therapy. Sci Rep 2018;8:1–11.
- Mehrabian H, Lam WW, Myrehaug S, Sahgal A, Stanisz GJ. Glioblastoma (GBM) effects on quantitative MRI of contralateral normal appearing white matter. J Neurooncol 2018;139:97–106.
- Chan RW, Chen H, Myrehaug S, Atenafu EG, Stanisz GJ, Stewart J, et al. Quantitative CEST and MT at 1.5T for monitoring treatment response in glioblastoma: early and late tumor progression during chemoradiation. J Neurooncol 2021;151:267–78.
- Chan RW, Myrehaug S, Stanisz GJ, Sahgal A, Lau AZ. Quantification of pulsed saturation transfer at 1.5T and 3T. Magn Reson Med 2019;82:1684–99.
- Desmond KL, Mehrabian H, Chavez S, Sahgal A, Soliman H, Rola R, Stanisz GJ. Chemical exchange saturation transfer for predicting response to stereotactic radiosurgery in human brain metastasis. Magnetic resonance in medicine. 2017 Sep;78(3):1110-20.
- Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ. Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res 2017;23:3667–75.
- Togao O, Yoshiura T, Keupp J, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-Oncology 2014; 16(3), 441–448.
- Zhou, J. (2011). Amide Proton Transfer Imaging of the Human Brain. Magnetic Resonance Neuroimaging. Methods in Molecular Biology (Clifton, N.J.), 711, 227–237.
- https://www.usa.philips.com/healthcare/education-resources/publications/fieldstrength/enhancing-brain-tumor-mri-with-apt