New study to learn from every patient treated with MR/RT

Focus interviews Kristina Orrling, Program Manager at Lygature, on how the study will guide the use of MR/RT to improve outcomes for cancer patients
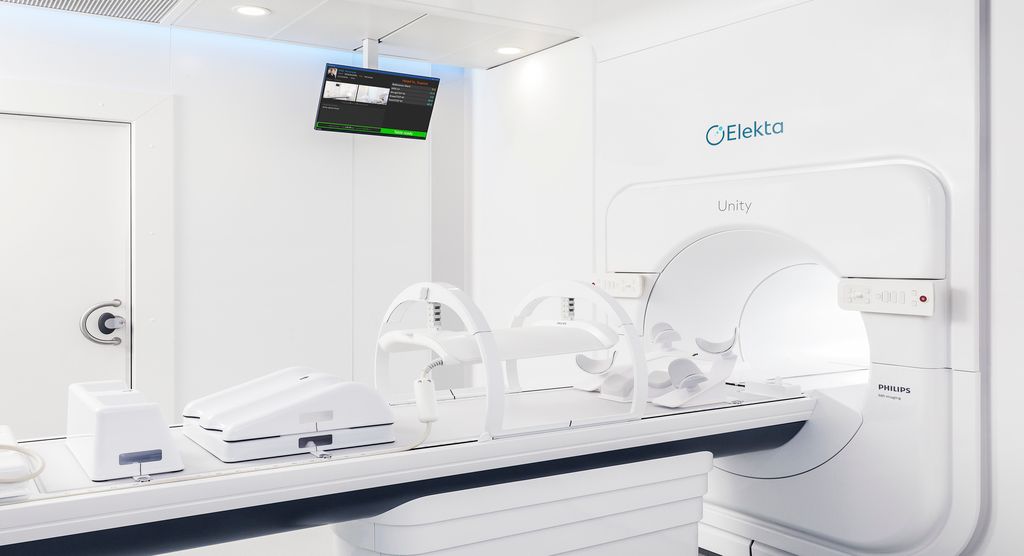
The international MR-Linac Consortium has launched the MOMENTUM study, the next step in the development of the Elekta Unity MR/RT system. The study is designed to generate data that enable safe, fast and, above all, ‘evidence-based’ introduction of magnetic resonance radiation therapy (MR/RT) into clinical practice.


The MOMENTUM study represents the next step in the development of the Elekta Unity MR/RT system and the study will be focused on building a robust body of real-world clinical evidence and insights made possible by this technology.
Kristine Orrling, Program Manager at Lygature, shares her experience on the MOMENTUM study and its progress with us.
Q: What is your role in the MOMENTUM project?
A: I am working for Lygature, a foundation aiming to accelerate the development of new medical solutions for patients by driving public-private collaboration between academia, industry and society and as an independent enabler in multi-stakeholder research partnerships.
Q: How would you describe MOMENTUM?
A: MOMENTUM is quite unique and exemplary for the MedTech industry and can be the blueprint for how to evaluate new medical technology in the clinic. It is the epitome of the collaborative initiative between Elekta—as the technical driver—and the forces of clinical partners from academia, grouped in the MR-Linac Consortium.
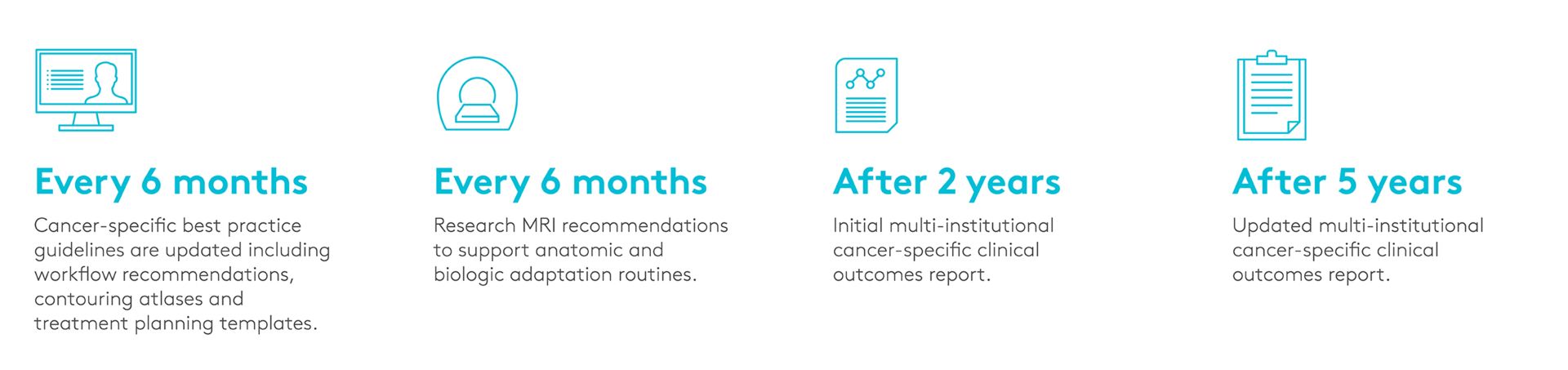
MOMENTUM creates a data repository for tracking outcomes of combining imaging and treatment of different cancer types. It will help to define, per tumor site, which patients can benefit the most from this approach.
Q: What is the value of the MOMENTUM study according to you?
A: Thanks to this project, different centers from different places in the world are now able to exchange pseudonymized data easily. This is the acceleration the technology needs to reach a high number of patients in each study cohort. This will facilitate and speed up the generation of proof points of the clinical outcomes. The data repository will support a virtuous learning cycle of standardization, assessment, optimization and then standardization again.
It also allows during the next five years to follow up in a very structured way the quality of life of patients in order to measure the gain.
Bringing leading parties together, MOMENTUM is a unique opportunity to learn how to use the technology in the best possible way and for Elekta to understand how to enhance the product. Additionally, as Elekta is a partner in MOMENTUM, the patient consent enables them to use the images and contours to develop Machine Learning algorithms to improve both the speed and accuracy of the automated segmentation, particularly in the online workflow.
It can be expected that the use of this data to drive changes in the technology will improve the quality of treatment, reduce the work required and reduce the time for each session thereby improving patient satisfaction and throughput.
Q: What is the latest status?
A: As of June 2019, MOMENTUM has seven Unity centers as part of the study and approximately 80 patients in the database. One month after the launch, University Medical Center (UMC) Utrecht—the first to start—has already included more than 10 patients. We expect the ‘momentum’ will grow fast as the other Unity centers go live and begin including their patients. For example, the first two centers in the U.S., Froedtert & the Medical College of Wisconsin (Wauwatosa, Wisconsin) and MD Anderson Cancer Center (Houston, Texas) currently have several patients enrolled.
Q: What are the key deliverables?
A: In addition to the generation of clinical studies evaluating treatment on toxicity and outcomes, one key deliverable is the clinical and technical profiles which will drive the standardization on how Elekta Unity could be used to help new users.
This will facilitate the implementation of some cost-effectiveness evaluation, providing evidence to the different countries government to assess whether this technology is a safe investment.
To learn more, Prof. Helena Verkooijen, MD, PhD, a PI for MOMENTUM, explains her views on MOMENTUM and the patient experience.

Elekta Unity is CE-marked and 510(k) cleared. Not commercially available in all markets.