First-of-its-kind program to reinforce MR-guided radiation therapy evidence base
Multi-center randomized phase II trials on prostate and rectal cancer are first to receive support from the Hypothesis Testing Program
Since the introduction of the Elekta Unity MR-Linac, clinical evidence for MR-guided radiation therapy (MRgRT) has been building every year since the modality’s introduction, thus significantly changing paradigms and clinical practice in radiotherapy. With high quality MR images and an integrated online adaptive workflow, clinicians, for the first time, are able to not only acquire crystal clear images of cancer targets and healthy tissues, but they also can adapt the delivery of radiation for every treatment session to account for daily and intra- and inter-fraction changes. Now, with the MR-Linac Consortium’s Hypothesis Testing Program (HTP), researchers will be empowered to build even more compelling evidence through funding support for large-scale randomized clinical trials.

“The HTP, focused exclusively on Elekta Unity, seeks to build on the volume of the platform’s clinical evidence and facilitate multi-institutional relationships between Unity users worldwide," says Veronica Dell’Acqua, MD, Director of Medical Affairs and Clinical Research. "To our knowledge, this program is the first-of-its-kind among medical technology companies, which is important because we truly believe that MRgRT is the future of radiation therapy. The HTP will enable clinicians using Unity to produce strong clinical evidence for the modality’s value."
Each HTP trial submission must be a clinical prospective study and its importance considered based on quality of life metrics, overall survival or efficiency advantages. The proposed trial also should be impractical to reproduce on any system other than the Elekta Unity MR-Linac and the research must be part of the MOMENTUM international registry on MRgRT.

Listen to Dr. Veronica Dell’Acqua, speak about the Hypothesis Testing Program.
Dr. Dell’Acqua adds that all of the more than 90 MR-Linac Consortium members can submit ideas for consideration to the HTP. An HTP committee of internal and external reviewers then evaluates each proposal.
Prostate and rectal cancer studies are first HTP trials
The first two HTP trials will evaluate the toxicity reduction effect of dose de-escalation in prostate radiotherapy and how MRgRT might help individuals with rectal cancer avoid life-altering surgery.
Multiple centers led by The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research (London, UK), the Netherlands Cancer Institute (Amsterdam, Netherlands) and Sunnybrook Health Sciences Centre (Toronto, Canada) were selected as the HTP-1 winner for their DESTINATION 2 prostate cancer study proposal using the MR-Linac in two fractions, and centers led by clinicians at Tübingen University Hospital (Tübingen, Germany) were chosen for their proposal exploring dose escalated radiotherapy for organ preservation in rectal cancer.
| Name | DESTINATION-2 | MARS |
| Objective | Establish if de-escalating the radiotherapy dose in the non-affected part of the prostate in two fractions with 0 PTV reduces toxicity | Generate evidence for MR-guided dose escalated radiotherapy for organ preservation in rectal cancer |
| Number of patients | ~200 | ~200 |
| Primary endpoint | Acute GU toxicity | 2-year total mesorectal excision (TME)-free survival |
| Treatment regimen | Arm 1 (uniform dose) will receive 27 Gy in 2 fractions to the whole
prostate + seminal vesicles (SV) CTV with 3 mm PTV margin Arm 2 (de-escalated dose) will use two dose levels. The whole prostate will receive 20 Gy in 2 fractions with a 0 mm PTV margin. The intraprostatic tumor masses (on MRI) + 4 mm margin will receive 27 Gy in 2 fractions. | Radiotherapy with standard of care 50 Gy (25 fractions, 2 Gy/fraction) plus weekly ≥3Gy boost to the GTV |
| Principal investigator | Alison Tree, MD, Consultant Clinical Oncologist at The Royal Marsden NHS
Foundation Trust and Institute of Cancer Research, UK Danny Vesprini, MD, Radiation Oncologist at Sunnybrook’s Odette Cancer Centre, Canada Prof. Dr. Uulke van der Heide, Group leader Imaging technology in radiotherapy, the Netherlands Cancer Institute, the Netherlands | Prof. Cihan Gani, MD, Radiation Oncologist, Tübingen University
Hospital, Germany. Gert Meijer, PhD, Medical Physicist, University Medical Center, Utrecht, the Netherlands William Hall MD, Professor of Radiation Oncology and Surgery, Medical College of Wisconsin, Milwaukee, WI |
DESTINATION 2 (Dose de-escalation in prostate radiotherapy using the MRL in two fractions)

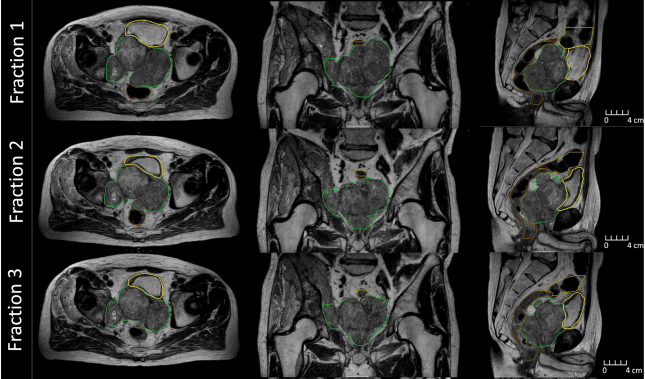
Following up on the HERMES1 and DESTINATION Elekta Unity trials, DESTINATION 2 will seek to establish if de-escalation of the radiotherapy dose in two fractions reduces toxicity. In the 200-patient study’s two arms, one arm gives 27 Gy to the whole prostate + 3 mm PTV, while a second arm delivers 27 Gy to the intraprostatic lesion + 4 mm with de-escalation to 20 Gy to the rest of the gland and no PTV margin.
“The standard of care in prostate cancer radiotherapy has always involved treating the whole gland to a high dose – there are not many other cancers where that’s the strategy,” says Danny Vesprini, MD, radiation oncologist at Sunnybrook’s Odette Cancer Centre. “Whereas in DESTINATION 2, we’re using the MR-Linac to de-escalate the dose away from the actual tumor we see on the MRI to try to minimize toxicity.”
Using a zero margin for the PTV is controversial, he adds, likely resulting in some nominal underdosing to the periphery of the prostate.
“Even if there is an increased chance that the patient’s PSA will increase in the future, the tradeoff is less toxicity, a better chance of keeping erections or less chance of urinary issues. Most patients would make that tradeoff in a heartbeat.”
“The philosophy of our approach is that as long as you’re treating the dominant lesion to a high dose, delivering a lower dose – though still considered an adequate microscopic radiation dose – away from the tumor is unlikely to compromise the chance of cure,” Dr. Vesprini says. “We know from other analyses and studies that the likelihood of dying of prostate cancer – for the type of patient that will enter this trial – is one percent or less, so de-escalating to the rest of the prostate is not going to impact survival. Even if there is an increased chance that the patient’s PSA will increase in the future, the tradeoff is less toxicity, a better chance of keeping erections or less chance of urinary issues. Most patients would make that tradeoff in a heartbeat.”
The precision of Elekta Unity is critical in this zero PTV, two-fraction study, demanding the ability to track the prostate’s position and adapt radiation in real time.
“If a gas bubble comes in and pushes the prostate two millimeters, you would be missing a huge amount,” he notes. “You need to have ways to stop the treatment if need be. So, it’s a matter of planning each day depending on the anatomy, and then while the treatment is ongoing, you need to be able to gate the treatment – otherwise you have to use a PTV margin.”
Using such a PTV a margin to account for uncertainty risks delivering more dose to the neurovascular bundles, the rectum and the bladder, increasing the likelihood of toxicity.
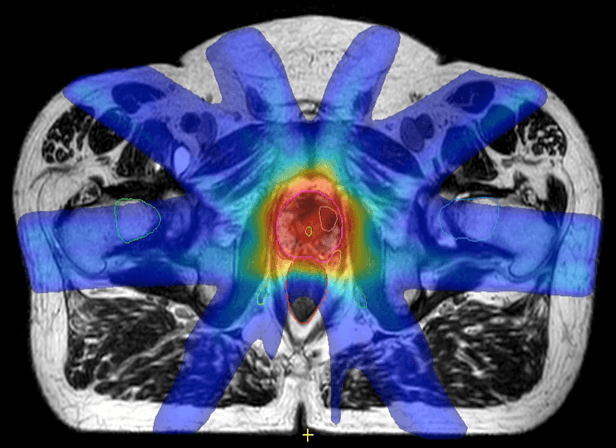
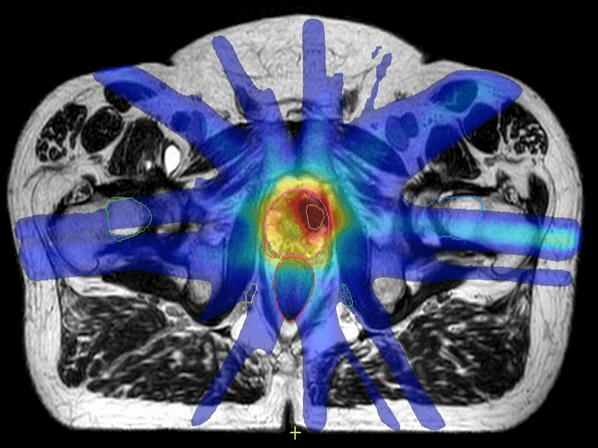
Image (left) shows that the hot dose (red) covers the whole prostate and some of the rectum and urethra. Image (right) shows the hot dose (red) delivered to the intraprostatic cancer only and a lower dose to the rest of the prostate. (Images courtesy of the Netherlands Cancer Institute, NKI).
“For this group of men, everything should be about toxicity,” Dr. Vesprini adds. “It’s not about life or death – we’re not going to improve the cure rate, since the biochemical control with modern SBRT is 95 percent in five years, although it’s probably going to be a bit lower at 10 years. Reducing toxicity is what this trial is focused on – harnessing this technology to make peoples’ lives better.”
MARS: MR-adaptive dose escalated radiotherapy in rectal cancer
Radical surgery to treat distal rectal cancer often can result in the patient needing a permanent colostomy, a quality of life detractor. However, clinicians at University Hospital Tübingen have been using their Elekta Unity MR-Linac to apply a precisely targeted high dose boost during conventional chemoradiation to increase clinical complete response rates, thus avoiding surgery for more patients.
Designated a winner of support through the HTP, the Tübingen group (HTP-2) and other centers will use the MR-adaptive dose escalated radiotherapy in rectal cancer (MARS) technique to build clinical evidence for the MRgRT approach. The results for the first five patients2 and an additional 13 have been very promising; only one patient has not achieved a clinical complete response and has been recommended for surgery.

“Patients are interested in MRgRT because we can offer a treatment alternative that promises a high quality of life and without lowering the chances of cure,” says University Tübingen radiation oncologist Prof. Cihan Gani, MD. “It’s a major advantage, as surgery in distal tumors often results in the removal of the entire rectum with the sphincter and placement of a colostomy that is most likely permanent.”
Delivering a very high boost dose is not feasible with any system other than Elekta Unity, he says. A CBCT linac lacks the imaging capability to clearly visualize rectal tumors, much less how they shrink and move throughout the treatment. As a result, the use of large margins is required to account for uncertainty.
“With Elekta Unity, we can deliver a high boost dose to the primary tumor in an adaptive manner – delineating the tumor on the days that we use this boost – thereby sparing the healthy parts of the rectum,” Prof. Gani says.
Individuals with rectal cancer who qualify for Tübingen’s MRgRT protocol are those with T1-T3-non-circumferential early stage tumors in the mid- or distal rectum.
The protocol that was developed by the Tübingen researchers applies radiotherapy with concomitant chemotherapy (5-FU) on a conventional linac radiotherapy to the primary tumor, the mesorectum and internal iliac lymph nodes (CTV1), with a seven to 10 mm PTV-margin. The CTV1 was treated with 1.8 Gy per fraction to a total of 45 Gy and a simultaneous integrated boost (SIB) to the primary tumor (CTV2) was applied with 2 Gy per fraction to a total of 50 Gy (CTV2).
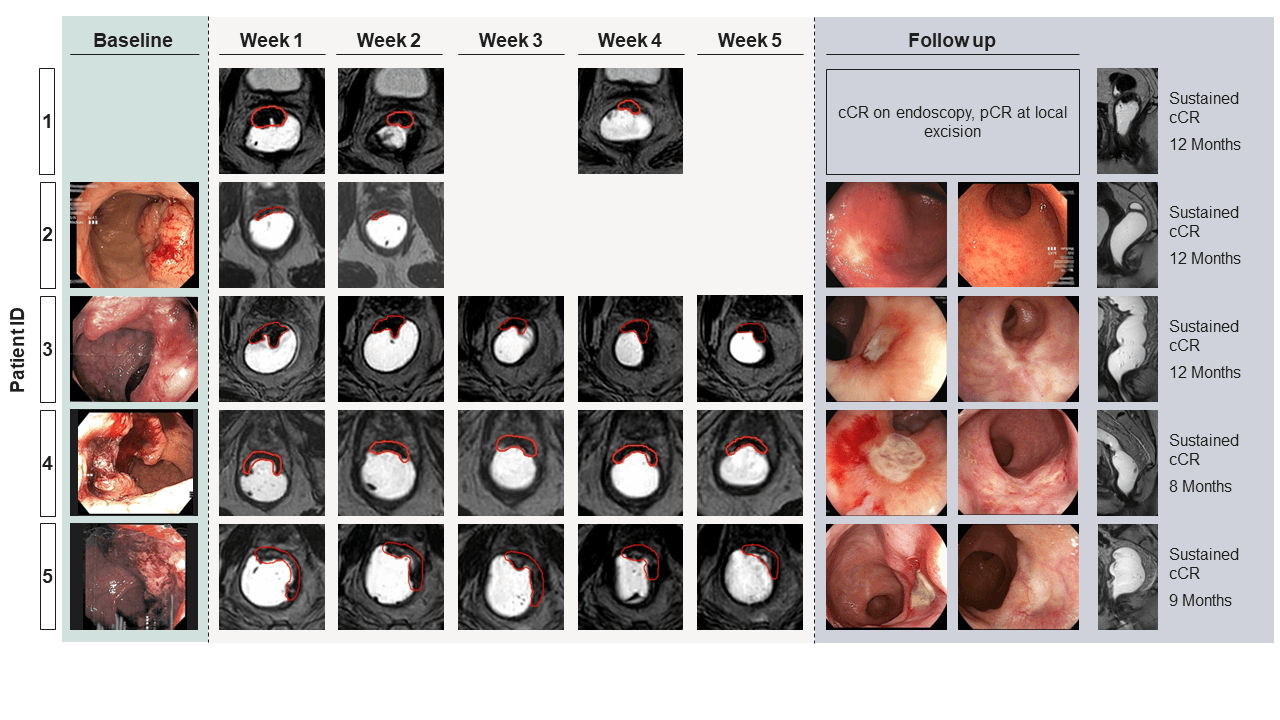
“In addition, patients were planned for a weekly response adaptive boost [sixth] fraction with 3 Gy per fraction on Elekta Unity,” Prof. Gani says. “A total of five boost fractions was scheduled, however in some cases of strong tumor shrinkage, we decided to discontinue the weekly boost fractions and continue with the fractionated treatment as the primary tumor had already vanished.”
Through HTP support, Tübingen and other participating centers will be able to generate more evidence within a clinical trial and help investigators define the optimal boost dose, he adds. “For now, we’re using 3 Gy per fraction and we want to explore whether we could use a higher dose and maintain low toxicity,” Prof. Gani observes. “And a trial allows us – within standardized inclusion and exclusion criteria – to prove the hypothesis that MRgRT does increase the clinical complete response rates in these patients, and results in good quality of life.”
Learn more about MR-Linac Consortium’s Hypothesis Testing Program.
References
- Westley RL, Biscombe K, Dunlop A, et al. Interim toxicity analysis from the randomized HERMES trial of 2- and 5-fraction magnetic resonance imaging-guided adaptive prostate radiation therapy. Int J Radiat Oncol Biol Phys. 2023 Sep 29:S0360-3016(23)07949-X. doi: 10.1016/j.ijrobp.2023.09.032. Epub ahead of print. PMID: 37776979.
- Boeke S, Uder L, Ehlers J, et al. Online MR guided dose escalated radiotherapy for organ preservation in distal rectal cancer. Clin Transl Radiat Oncol. 2022 Oct 15;37:153-156. doi: 10.1016/j.ctro.2022.10.003. PMID: 36339638; PMCID: PMC9630771.
LARMRL240130