Evolution of prostate radiotherapy accelerates with Elekta Unity MR-Linac
From 20 fractions to two: The Royal Marsden NHS Foundation Trust’s Dr. Alison Tree reviews technology-driven advances in the treatment of prostate cancer
With 1.5 million prostate cancers diagnosed each year worldwide1 and the disease representing about 25 percent of radiation oncology departments’ workload, there is an imperative to improve prostate radiotherapy by increasing its efficacy and safety.

Several clinical trials demonstrate that prostate cancer radiotherapy is heading in one direction: fewer fractions, higher doses and, with increasing evidence, acceptable toxicity. From the 20-fraction standard of care adopted after the CHHiP2 study, the PACE B3 trial’s five-fraction schedule and two trials involving Elekta Unity (one of which tests just two fractions), prostate radiotherapy has fundamentally transformed. Below, Alison Tree, MD, Consultant Clinical Oncologist at The Royal Marsden, reviews these developments.
“First, the prostate moves within the patient, so without excellent image guidance you’re going to miss the target some days unless you put large margins around the gland,” she says. “Second, we encountered big problems with bowel toxicity, which could persist or arise years after their treatment.”
Lacking advanced image guidance, clinicians used bone imaging as a surrogate prostate position, which is an extremely inaccurate way to target the organ. However, the development of a cone-beam CT workflow integrated in linacs (e.g., Elekta Synergy®) and other technological innovations, changed the treatment landscape and triggered several trials aimed at increasing doses and reducing the number of fractions.
“It’s hard to overstate the progress we’ve made in the way we treat prostate cancer with radiation over the last 15 years.”
“It’s hard to overstate the progress we’ve made in the way we treat prostate cancer with radiation over the last 15 years,” Dr. Tree observes. “We’ve discovered that if we give more dose and do it safely we can cure more cancer. There was also the realization that prostate cancer differs from many other cancers in how it responds to radiation [i.e., its low alpha/beta ratio] and we’re just as likely to cure it if we give a smaller number of bigger doses.”
Studies demonstrate feasibility of hypofractionation
Dr. Tree cites The Royal Marsden /The Institute of Cancer Research’s (ICR) CHHiP2 study, which showed that 3 Gy delivered over 20 fractions should become the standard of care.
“That’s important because it makes the patient’s life better – they don’t have to visit the hospital for eight weeks, it’s just four weeks,” Dr. Tree says. “The next step was to see if we could hypofractionate even further while limiting toxicity. The first results of the ongoing PACE B3 trial – comparing conventional fractionation with prostate SBRT delivered with 36.5 Gy in only five fractions – suggest that this short treatment course can be delivered with low levels of gastrointestinal or genitourinary acute toxicity. The HYPO-RT4 and PROFIT5 hypofractionation trials showed similar results.”

She adds that linac technological innovations, including cone-beam CT, fiducials and, lately, the integrated MR guidance in Elekta Unity are crucial to these successes.
“With better prostate visualization, you can get away with fewer fractions with more precision and fewer side effects,” Dr. Tree says. “What we’re seeing from the trials is that today we very rarely see bowel toxicity. However, bladder toxicity remains an issue we want to solve.”
Better imaging in the pre-Elekta Unity age helped Dr. Tree’s team (DELINEATE6 trial) and another group (FLAME7 trial) test the safety profile of dose escalation to the intraprostatic cancer zone only.
“DELINEATE looked at both the standard fractionation the FLAME group used and also doing a focal boost [67 Gy] with moderate hypofractionation and we’ve shown that that can equally be well-delivered with no more toxicity than you’d expect,” she notes. “The five-year outcome will show incredibly good cancer control.”
Elekta Unity: the ultimate in prostate visualization
The Royal Marsden started using the Elekta Unity MR-Linac in 2018 and began recruiting patients to participate in the PRISM8 trial (see FOCUS article). PRISM was a 30-patient Royal Marsden study to assess the technical feasibility of delivering moderately hypofractionated (3 Gy X 20) radiotherapy for prostate cancer using Elekta Unity, including the feasibility of changing the radiotherapy plan on a daily basis to mirror internal anatomy changes.
“MRI gives us a much better depiction of the prostate than does CT, which struggles to discern complex prostate anatomy,” Dr. Tree comments. “Elekta Unity enables me to contour the prostate capsule for planning purposes, while the cine MRI pulse sequence allows us to easily track target motion and surrounding organs during beam delivery.”
The PRISM study authors noted that “for patients with OARs abutting target volumes on their reference image we have demonstrated the potential for a target dose coverage improvement for MRgART (MR-guided adaptive radiotherapy) compared to C-arm linac treatment8.”
On to ultra-hypofractionation
The Royal Marsden /ICR investigators recently began the HERMES9 (Hypofractionated Expedited Radiotherapy for Men with Localized Prostate Cancer ) Elekta Unity trial. HERMES is a single-center phase II randomized study of ultra-hypofractionated SBRT in these patients.
“In HERMES, we’re asking whether five is the magic number of fractions as per PACE B, or can we go below five and make the treatments just as effective with improved convenience and maybe even reduce side effects,” Dr. Tree says.
One arm of the MRgART study gives 36.25 Gy in five fractions over 10 days, with a boost to 40 Gy over the tumor/prostate CTV. The experimental arm gives 24 Gy in two fractions over eight days, with a boost to 27 Gy over the tumor CTV.
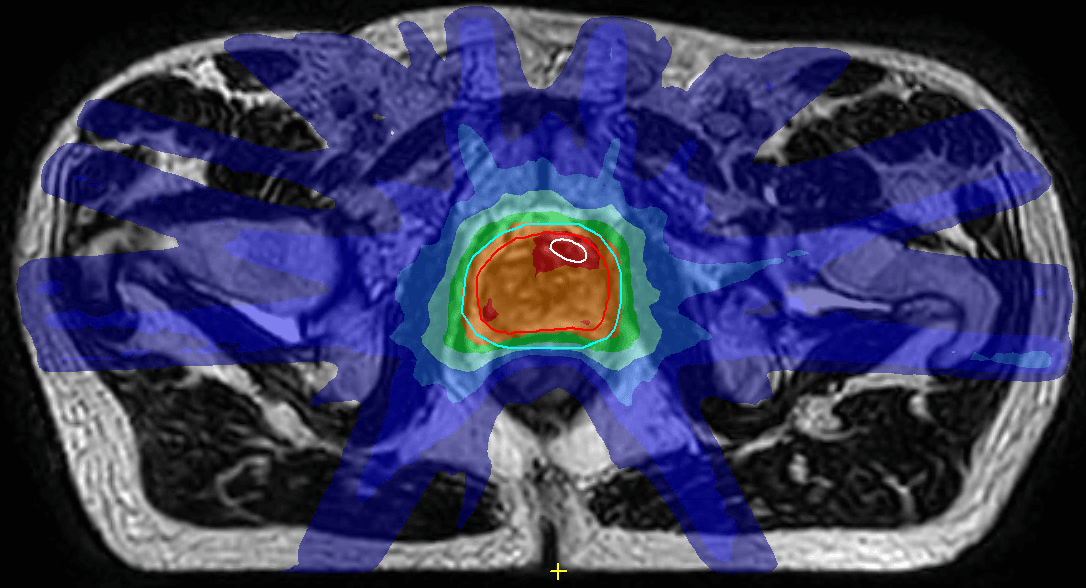
“We’ve recruited just less than half of the patients, but so far the two-fraction patients have done extraordinarily well,” she notes. “The first patient had practically no side effects at all from his treatment.” (Figure 1)
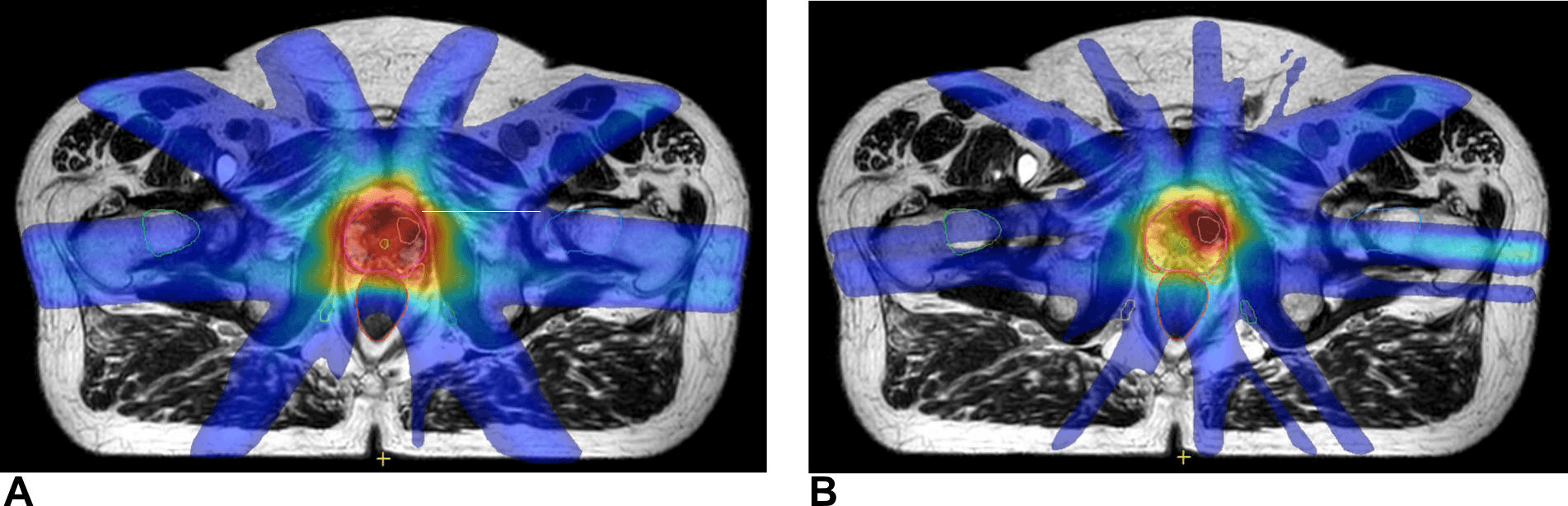
A subsequent multicenter trial hopes to explore whether Elekta MR-Linac Consortium researchers can combine two-fraction MRgART treatments with de-escalation to the non-tumor containing prostate.
“We will still be giving some dose to the whole prostate, so this is not truly focal therapy, but we’re trying to achieve the best therapy ratio between side effects and efficacy,” Dr. Tree explains. “The prostate is surrounded by numerous important structures, the bladder, rectum, vessels and nerves responsible for erectile function, in addition to the urethra, which goes through the middle of the gland. We will target the hot dose over the cancer and give a lower dose to the rest of the prostate. (see Figure 2)
She estimates that approximately 200 patients will participate in the de-escalation trial; 176 evaluable patients would be able to show a halving of genitourinary toxicity, i.e., a drop in genitourinary toxicity from the 25 percent in the PACE B trial down to 12.5 percent in this study.
“The key objectives in this trial are to reduce toxicity and improve the quality of life for our patients,” Dr. Tree asserts. “We want to reduce time on the bed for the patient. Many of them have loved the idea that they can come just twice for radiotherapy and we want to improve cost-effectiveness and efficiency for our hospitals. We just need to make sure that the MR-Linac has sufficient throughput to be a realistic proposition for most departments.”
Four years of “seeing what you treat”
Since 2018, Dr. Tree and her colleagues have enjoyed the power to see – in real-time – the cancer targets they’re treating, a capability that did not exist before Elekta Unity. In addition to prostate cancer, The Royal Marsden clinicians have treated rectal, bladder, gynecological, oligometastatic and head-and-neck cancers with their MR-Linac. The center is starting a pancreas program and will begin treating livers in the immediate future.
While Elekta Unity is applicable to many different cancers throughout the body, the MR-Linac seems tailor-made for prostate radiotherapy.
“Elekta Unity is a lovely machine to treat the prostate with because visualization is pristine.”
“Elekta Unity is a lovely machine to treat the prostate with because visualization is pristine,” she says. “I can adapt radiotherapy each day and I can make sure the treatment plan I deliver each day is optimal for that day’s anatomy, not just the average anatomy for that patient. And with Elekta Unity we also avoid the need for fiducials, which is uncomfortable for the patient. We find that lots of patients like being on the machine and consider it a positive experience. Elekta Unity is a really important tool in our toolbox for treating prostate cancer.”
Learn more about Elekta Unity.
References
- American Cancer Society 2022.
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016 Aug;17(8):1047-1060. doi: 10.1016/S1470-2045(16)30102-4. Epub 2016 Jun 20. Erratum in: Lancet Oncol. 2016 Aug;17 (8):e321. PMID: 27339115; PMCID: PMC4961874.
- Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019 Nov;20(11):1531-1543. doi: 10.1016/S1470-2045(19)30569-8. Epub 2019 Sep 17. PMID: 31540791; PMCID: PMC6838670.
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019 Aug 3;394(10196):385-395. doi: 10.1016/S0140-6736(19)31131-6. Epub 2019 Jun 18. PMID: 31227373.
- Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017; 35: 1884-90.
- Murray JR, Tree AC, Alexander EJ, et al. Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Nodules in Localized Prostate Cancer: Efficacy and Toxicity in the DELINEATE Trial. Int J Radiat Oncol Biol Phys. 2020 Mar 15;106(4):715-724. doi: 10.1016/j.ijrobp.2019.11.402. Epub 2019 Dec 5. PMID: 31812718.
- Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. 2021 Mar 1;39(7):787-796. doi: 10.1200/JCO.20.02873. Epub 2021 Jan 20. PMID: 33471548.
- Dunlop A, Mitchell A, Tree A, et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Transl Radiat Oncol. 2020 Apr 29;23:35-42. doi: 10.1016/j.ctro.2020.04.011. PMID: 32395640; PMCID: PMC7210377.
- Westley R, Hall E, Tree A. HERMES: Delivery of a Speedy Prostate Cancer Treatment. Clin Oncol (R Coll Radiol). 2022 Jul;34(7):426-429. doi: 10.1016/j.clon.2022.01.003. Epub 2022 Jan 31. PMID: 35093251; PMCID: PMC8802653.
LPRMRL220819