mDIBH and intrafraction IGRT in lung SBRT
Case: Lung metastases.
Facility: Radiotherapy Center, Sichuan Cancer Hospital, Chengdu, Sichuan, China.
Contributors: Zhang Peng, chief physician. Huang Rui, attending physician. Li Jie, Yao Xinghong, Liao Xiongfei and Liu Min, medical physicists. Ying Wei, Wang Hui, Wang Shoulong and Liu Lihao, technicians.
Overview
In a moderate deep inspiration breath-hold (mDIBH), the patient holds their breath at a pre-set deep inspiration threshold during treatment delivery, according to their individual situation. The mDIBH increases lung volume and expands the thorax, while keeping the tumor static and a stable distance from surrounding normal tissues. This technique can also be used to increase the distance between the tumor and the heart in certain cases. These factors make this technique particularly beneficial for breast and lung treatments, effectively reducing dose to critical organs, such as healthy lung and heart.
Sichuan Cancer Hospital’s radiotherapy department is equipped with Elekta Infinity and Elekta’s active respiratory control system, Active Breathing Coordinator™ R3.0 (ABC). This system uses an air valve to monitor respiration and it calculates inspiratory flow through an airflow detector. The patient’s respiratory curve is shown on a screen. When the pre-set threshold is reached, the patient pushes the green enable button and a staff member presses the control button to activate the air valve. The patient’s breathing stops temporarily, and the lung volume remains unchanged. The ABC breath-hold signal is transferred to the linac by Elekta’s Response™ gating interface, turning the beam on automatically. When the breath-hold is completed, or the patient releases the green enable button, the patient is able to breath freely again and the beam stops. This process is repeated until the mDIBH radiotherapy treatment is completed.
Sichuan Cancer Hospital’s Radiotherapy Center applies mDIBH and intra-fraction image guidance technology to deliver SBRT in patients with lung metastases, as illustrated in the following case studies:
Patient 1:
A 43-year-old female patient was diagnosed with hypopharyngeal carcinoma with cervical lymph node metastasis in April 2017 and was treated with a combination of chemotherapy and radiotherapy. Re-examination in August 2019 revealed right lung metastases located at the inner side of the right middle lobe (lesion 1) and in the base section of the right lower lobe (lesion 2). Lesion 1 was located close to the heart. Lesion 2 was >1 cm in size and had a large motion amplitude (~2 cm) due to respiratory movement. Since the patient had good lung function, a stable breathing cycle, and was able to maintain a breath-hold according to instructions, it was decided to treat these metastases using mDIBH SBRT. Prescribed doses were 50 Gy in 5 fractions (one fraction every other day) to lesion 1: PGTV1; and 50 Gy in 5 fractions (one fraction every other day) to lesion 2: PGTV2.
Patient 2:
A 45-year-old male patient was diagnosed with cancer of the esophagus in May 2014. Following esophagectomy, the cancer was diagnosed as poorly-differentiated squamous cell carcinoma (PDSCC), pT3N1M0, stage II, in the mid-thoracic region and the patient underwent chemotherapy. Re-examination in April 2017 revealed a cervical lymph node metastasis, which was treated using radical radiotherapy. Re-examination in August 2019 revealed lung metastases. Two bigger lesions were located in the right lung: at the back of the lower lobe (lesion 1) and in the upper lobe (lesion 2). Both lesions were >1 cm in size and had a movement range of around 1.5-2 cm. Additional lesions were scattered in both lungs, impacted greatly by respiratory movement. The patient’s respiratory motion baseline position was not stable.
In this case, due to baseline drift and respiratory movement amplitude, there is a risk of missing the target if the patient is treated while free-breathing, even with a PTV margin of >2 cm. Furthermore, following esophagectomy, there is risk of an adverse reaction, such as lung fibrosis, with this SBRT. Based on these factors, combined with the patient’s good lung function, stable breath and compliant breath-hold, it was decided to treat the patient using mDIBH SBRT. Prescribed doses were: 50 Gy in 5 fractions (one fraction every other day) to lesion 1: PGTV1; and 50 Gy in 5 fractions (one fraction every other day) to lesion 2: PGTV2.
Treatment planning
CT simulation
Both patients were immobilized using BodyFIX to minimize the impact of patient movement during treatment. Prior to setup, patients received mDIBH coaching using ABC and their mDIBH threshold was set, based on their respiratory status and duration.
With ABC, patients can see their real-time respiratory range and threshold while lying on the couch. They can control the deep breath until the threshold is met and the breath-hold. With the patient in breath-hold, a lead point is placed to mark the CT grid origin. The breath-hold is repeated and the CT scan is captured with the patient in mDIBH. One breath-hold is required for one CT scan.
In order to better assess the benefits of mDIBH, CT images were also captured with the patients in free-breathing. The two sets of images (figure 1) were transferred to Monaco version 5.11. Breath-hold details and lung volumes for both patients are shown in table 1.
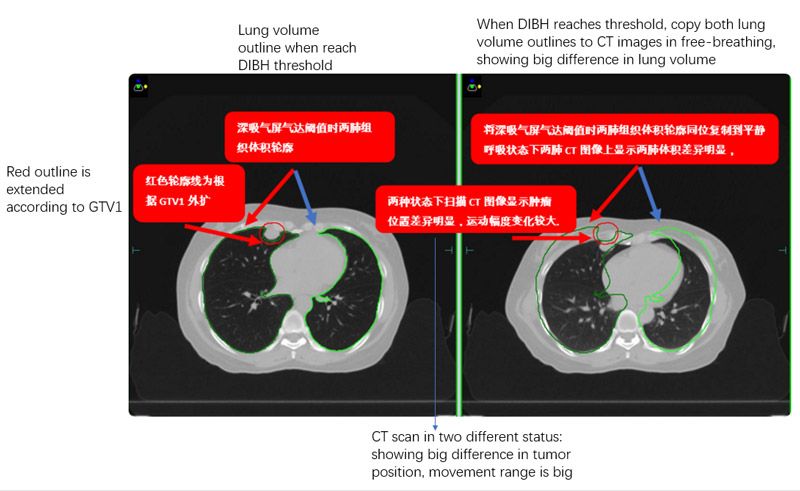
Table 1. Breath-hold details for both patients
| Inspiration threshold | Breath-hold duration | V(lung) at free breathing | V(lung) at breath-hold | V(lung) increase | |
| Patient 1 | 1.4 L | 25 s | 3443.1 cc | 5155.7 cc | 49.7% |
| Patient 2 | 1.2 L | 25 s | 4410.8 cc | 4931.7 cc | 11.8% |
Plan details
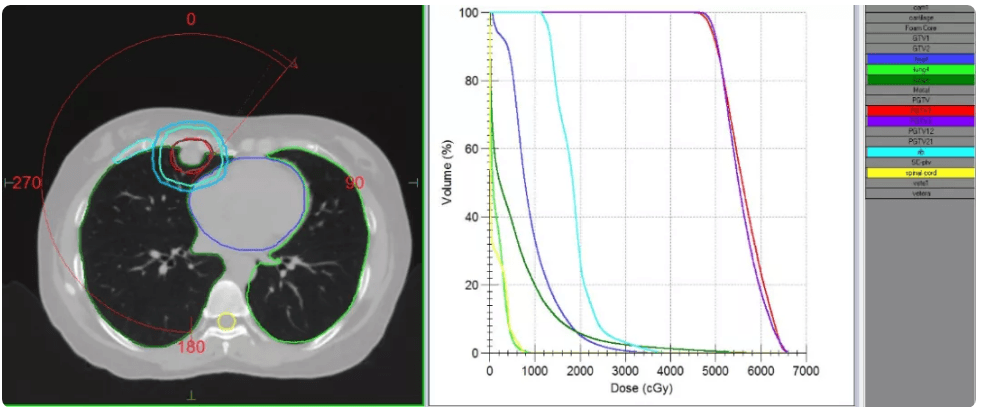
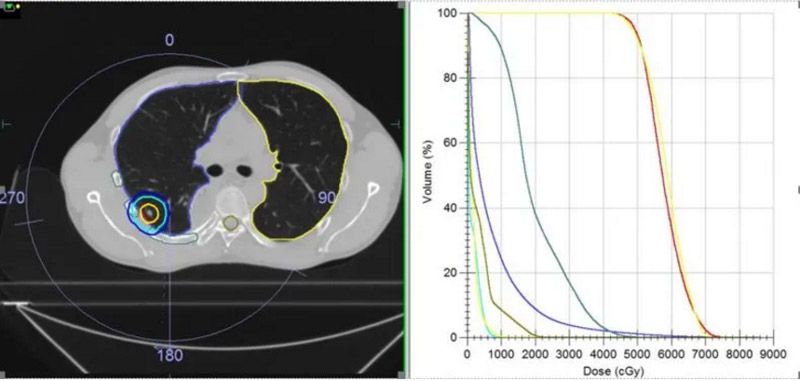
Treatment plans for both patients were created for high dose SBRT with mDIBH, delivered using Elekta Infinity with the Agility MLC. To improve treatment efficiency, and reduce breath-hold duration and frequency, both patients were treated using a single iso-center 6 MV FFF (high dose rate 1400MU/minute) VMAT plan, together with Monaco’s dual arc function, which increased plan quality and shortened treatment times.
Plans were evaluated according to TG101 critical structure dose constraints (table 2). With patients in mDIBH, the targets were static. Following discussions between the physicians and physicists, the CTVs were expanded by 5 mm to obtain PTVs (red outline in figures 2 and 3), in order to best protect the surrounding normal tissues. The plans were confirmed by the physician and verified using ArcCheck. The Gamma Analysis pass rate was around 95% (3 mm, 2%).
Table 2. Dose targets and constraints for both patients.
| Patient | Target / OAR | Clinical prescription requirements | Real plan result | Total MU (delivery time) |
| Patient 1 | PGTV1 | 5000 cGy/5# to cover 95% volume | 5000 cGy/5# covered 90.92% volume | 4029.19 (185.55s) |
| PGTV2 | 5000 cGy/5# to cover 95% volume | 5000 cGy/5# covered 92.99% volume | ||
| Full lung | V12.5<1500 cc, V13.5<1500 cc | V12.5=389.78 cc, V13.5=343.53 cc | ||
| Heart | Dmax<3800 cGy, V32<15 cc | Dmax=3853.1 cGy, V32=1.463 cc | ||
| Rib | Dmax<4300 cGy | Dmax=3844.8 cGy | ||
| Great vessels | Dmax<5300 cGy, V47<15 cc | Dmax=5035.5 cGy, V47=0.071 cc | ||
| Spinal cord | Dmax<3000 cGy | Dmax=807.8 cGy | ||
| Patient 2 | PGTV1 | 5000 cGy/5# to cover 95% volume | 5000 cGy/5# covered 93.48% volume | 7086.82 (362.12 s) |
| PGTV2 | 5000 cGy/5# to cover 95% volume | 5000 cGy/5# covered 92.23% volume | ||
| Full lung | V12.5<1500 cc, V13.5<1500 cc | V12.5=447.17 cc, V13.5=403.56 cc | ||
| Heart | Dmax<3800 cGy, V32<15 cc | Dmax=960.9 cGy, V32=0 cc | ||
| Rib | Dmax<4300 cGy, V35<1 cc | Dmax=5506 cGy, V35=2.6 cc | ||
| Liver | V21<700 cc | V21=0.21 cc | ||
| Spinal cord | Dmax<3000 cGy, V23<0.35 cc, V14.5<1.2 cc | Dmax=2351 cGy, V23=0.005 cc, V14.5=1.9 cc |
Treatment Delivery
Before treatment, both patients were re-positioned in mDIBH using ABC. The isocenter was marked using a tattoo on the patients’ skin. 3D CBCT XVI images were captured under DIBH, and required positioning corrections were made. During treatment, patients were assisted in mDIBH using ABC and the beam was controlled automatically using the Response gating interface, this protecting patients from accidental doses. The breath-hold process was repeated until the required dose was delivered.
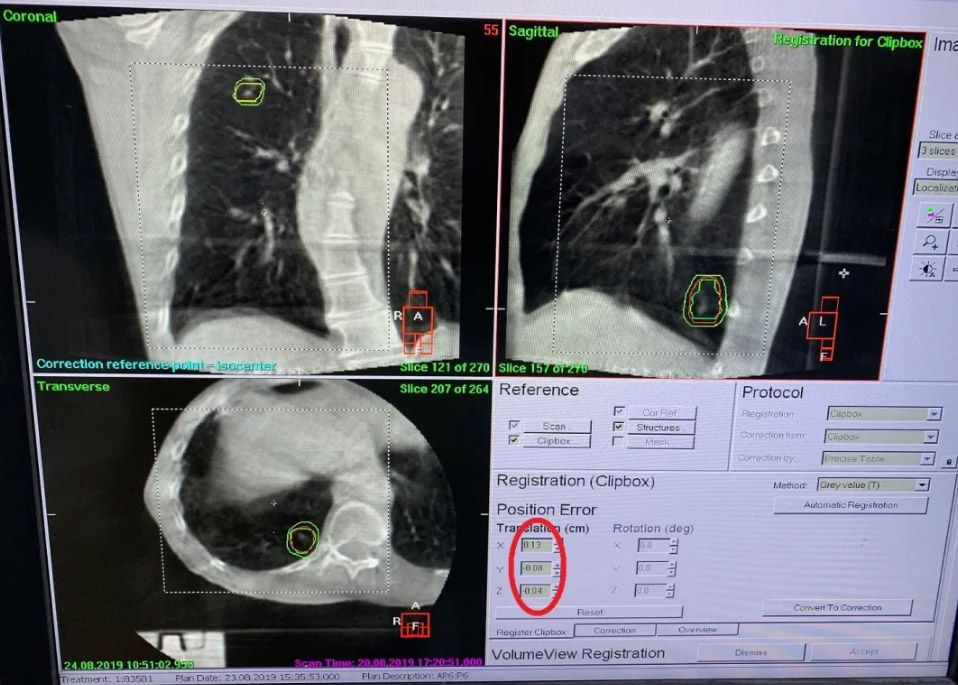
In order to check patient position during treatment, intra-fraction CBCT imaging was performed. Appropriate XVI image acquisition parameters were set before treatment, according to each plan’s machine parameters. When implementing the first arc, intra-fraction 3D CBCT images were acquired at the same time to monitor tumor position errors/changes during treatment. Both patients’ intra-fraction position errors (X, Y, Z) were within tolerance (1.5 mm) (figure 4).
Discussion and conclusions
Both lung metastases patients received lung SBRT, using Monaco VMAT treatment planning, BodyFIX patient positioning, mDIBH with ABC, Elekta Infinity with Agility, Response gating interface, XVI MotionView and XVI intra-fraction 3D CBCT image guidance.
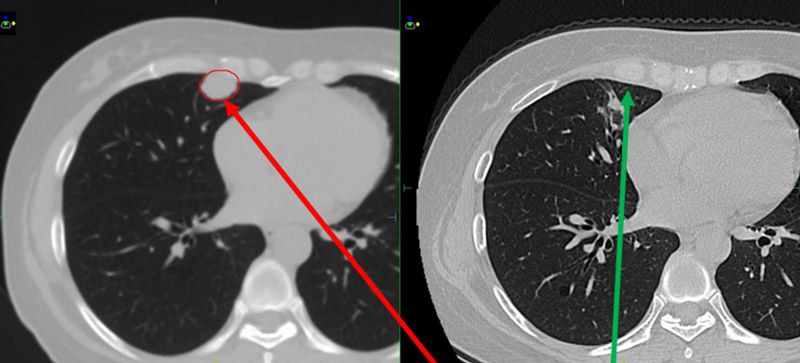
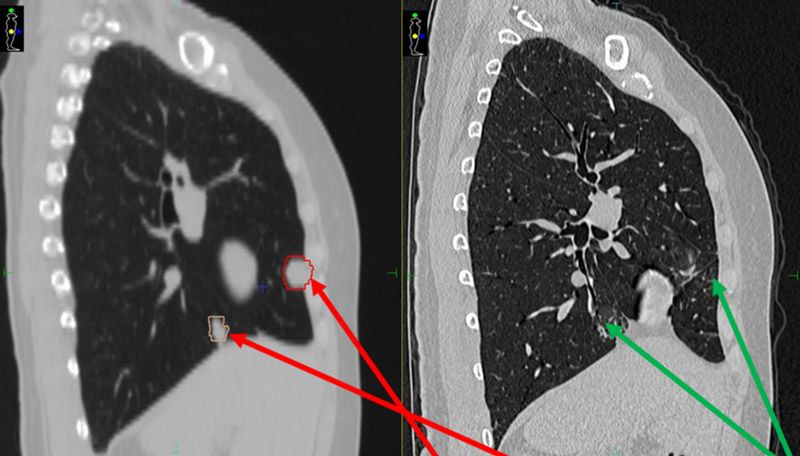
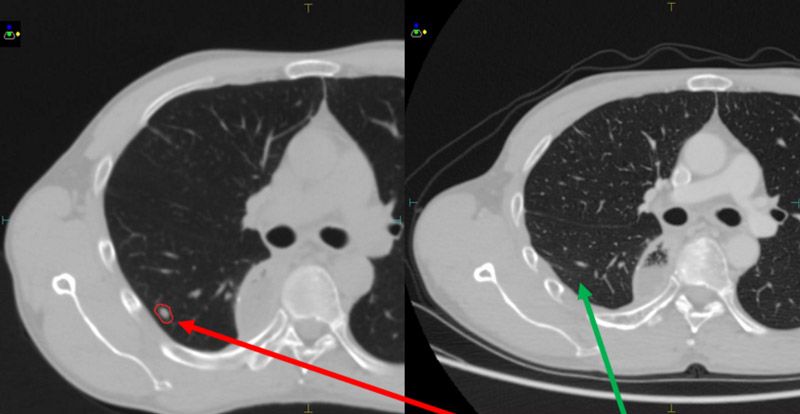
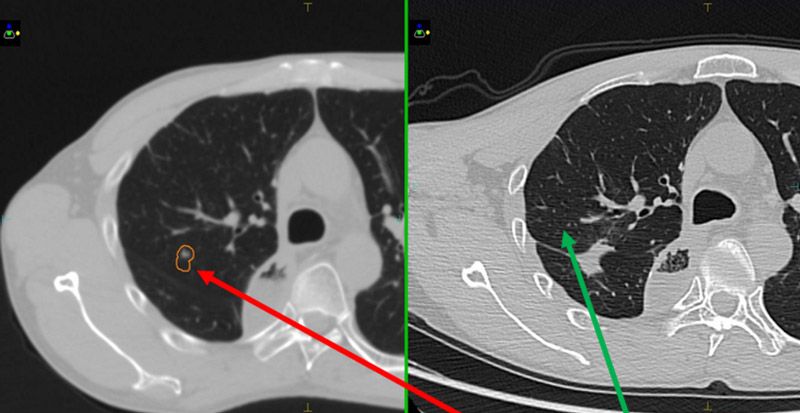
Seven months following treatment, CT images for both patients show that all targets have disappeared (figures 5-8), demonstrating complete remission and a positive treatment effect. There were no obvious toxicities or side effects. Compared to free-breathing, mDIBH largely reduced the PTV volume, increased the accuracy of dose delivery to the targets, expanded lung volume and increased the distance between targets and heart. This effectively reduced unnecessary doses to the lungs and heart, and reduced toxicity and side effects for the patients.